BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes
- PMID: 25534037
- PMCID: PMC4306630
- DOI: 10.1007/s11745-014-3981-9
BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes
Abstract
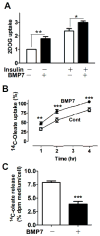
Adult humans have a substantial amount of inducible-brown (or beige) fat, which is associated with increased energy expenditure and reduced weight gain via thermogenesis. Despite the identification of key regulators of beige adipogenesis, impacts of dietary factors on adaptive thermogenesis are largely unknown, partly due to a lack of validated human cell models. Bone morphogenetic protein 7 (BMP7) is known to promote brown adipogenesis in rodent and human progenitor cells. However, controversy still surrounds the cellular identity in BMP7-mediated transition of white to brown adipocytes. The aim of this study was to confirm BMP7-derived human adipocytes as a relevant in vitro model of human beige adipocyte by verifying the cellular lineage and metabolic activity. In this study, we hypothesized that pre-exposure of the stromal vascular (SV) fraction of primary human adipogenic precursor cells (hASC) to BMP7 would convert metabolically active brown adipocytes. Our results showed that exposure of hASC to human BMP7 was associated with significant escalation of (1) UCP1 gene expression, a signature gene of brown adipocytes, (2) beige specific marker gene expression (i.e., CD137 and TMEM26), (3) glucose and fatty acid uptake, and (4) basal and cAMP-stimulated oxygen consumption rate compared to white adipocyte control. Taken together, we demonstrated that BMP7 mediates conversion of hASC into metabolically active beige adipocytes. By confirming the cellular identity and metabolic activity, this BMP7-induced human beige adipocytes from hASC should aid in the discovery and assessment of bioactive molecules to promote adaptive thermogenesis.
Conflict of interest statement
None
Figures



Similar articles
-
BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells.Am J Physiol Cell Physiol. 2014 Mar 1;306(5):C431-40. doi: 10.1152/ajpcell.00290.2013. Epub 2013 Nov 27. Am J Physiol Cell Physiol. 2014. PMID: 24284793
-
Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes.Am J Physiol Endocrinol Metab. 2014 Feb 15;306(4):E363-72. doi: 10.1152/ajpendo.00119.2013. Epub 2013 Dec 17. Am J Physiol Endocrinol Metab. 2014. PMID: 24347060
-
Functional thermogenic beige adipogenesis is inducible in human neck fat.Int J Obes (Lond). 2014 Feb;38(2):170-6. doi: 10.1038/ijo.2013.82. Epub 2013 May 21. Int J Obes (Lond). 2014. PMID: 23736373 Free PMC article.
-
[Evaluation of Naturally Occurring Compounds Regulating Brown/Beige Adipocyte Differentiation].Yakugaku Zasshi. 2019;139(6):861-866. doi: 10.1248/yakushi.18-00173-3. Yakugaku Zasshi. 2019. PMID: 31155526 Review. Japanese.
-
UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes.Front Endocrinol (Lausanne). 2020 Jul 28;11:498. doi: 10.3389/fendo.2020.00498. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32849287 Free PMC article. Review.
Cited by
-
Inhibitory Effects of Toll-Like Receptor 4, NLRP3 Inflammasome, and Interleukin-1β on White Adipocyte Browning.Inflammation. 2018 Mar;41(2):626-642. doi: 10.1007/s10753-017-0718-y. Inflammation. 2018. PMID: 29264745 Free PMC article.
-
Adipose tissue and age‑dependent insulin resistance: New insights into WAT browning (Review).Int J Mol Med. 2021 May;47(5):71. doi: 10.3892/ijmm.2021.4904. Epub 2021 Mar 11. Int J Mol Med. 2021. PMID: 33693956 Free PMC article. Review.
-
Regulation of Energy Expenditure and Brown/Beige Thermogenic Activity by Interleukins: New Roles for Old Actors.Int J Mol Sci. 2018 Aug 29;19(9):2569. doi: 10.3390/ijms19092569. Int J Mol Sci. 2018. PMID: 30158466 Free PMC article. Review.
-
Comparative analysis of human UCB and adipose tissue derived mesenchymal stem cells for their differentiation potential into brown and white adipocytes.Mol Biol Rep. 2018 Jun;45(3):233-244. doi: 10.1007/s11033-018-4156-1. Epub 2018 Feb 16. Mol Biol Rep. 2018. PMID: 29453764
-
Beyond the bone: Bone morphogenetic protein signaling in adipose tissue.Obes Rev. 2019 May;20(5):648-658. doi: 10.1111/obr.12822. Epub 2019 Jan 4. Obes Rev. 2019. PMID: 30609449 Free PMC article. Review.
References
-
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. - PubMed
-
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. - PubMed
-
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. - PubMed
-
- Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

