Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910
- PMID: 25534388
- PMCID: PMC4302214
- DOI: 10.1200/JCO.2014.58.0662
Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910
Abstract
Purpose: To determine whether prolonged androgen suppression (AS) duration before radiotherapy improves survival and disease control in prostate cancer.
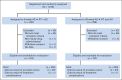
Patients and methods: One thousand five hundred seventy-nine men with intermediate-risk prostate cancer were randomly assigned to 8 weeks of AS followed by radiotherapy with an additional 8 weeks of concurrent AS (16 weeks total) or to 28 weeks of AS followed by radiotherapy with an additional 8 weeks of AS (36 weeks total). The trial sought primarily to detect a 33% reduction in the hazard of prostate cancer death in the 28-week assignment. Time-to-event end points are reported for up to 10 years of follow-up.
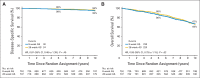
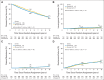
Results: There were no between-group differences in baseline characteristics of 1,489 eligible patients with follow-up. For the 8- and 28-week assignments, 10-year disease-specific survival rates were 95% (95% CI, 93.3% to 97.0%) and 96% (95% CI, 94.6% to 98.0%; hazard ratio [HR], 0.81; P = .45), respectively, and 10-year overall survival rates were 66% (95% CI, 62.0% to 69.9%) and 67% (95% CI, 63.0% to 70.8%; HR, 0.95; P = .62), respectively. For the 8- and 28-week assignments, 10-year cumulative incidences of locoregional progression were 6% (95% CI, 4.3% to 8.0%) and 4% (95% CI, 2.5% to 5.7%; HR, 0.65; P = .07), respectively; 10-year distant metastasis cumulative incidences were 6% (95% CI, 4.0% to 7.7%) and 6% (95% CI, 4.0% to 7.6%; HR, 1.07; P = .80), respectively; and 10-year prostate-specific antigen-based recurrence cumulative incidences were 27% (95% CI, 23.1% to 29.8%) and 27% (95% CI, 23.4% to 30.3%; HR, 0.97; P = .77), respectively.
Conclusion: Extending AS duration from 8 weeks to 28 weeks before radiotherapy did not improve outcomes. A lower than expected prostate cancer death rate reduced ability to detect a between-group difference in disease-specific survival. The schedule of 8 weeks of AS before radiotherapy plus 8 weeks of AS during radiotherapy remains a standard of care in intermediate-risk prostate cancer.
Trial registration: ClinicalTrials.gov NCT00005044.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Personalizing the duration of androgen-deprivation therapy use in the management of intermediate-risk prostate cancer.J Clin Oncol. 2015 Feb 1;33(4):301-3. doi: 10.1200/JCO.2014.59.0968. Epub 2014 Dec 22. J Clin Oncol. 2015. PMID: 25534385 No abstract available.
-
[Recommended duration of androgen suppression of localized pancreatic cancer before radiotherapy is still uncertain].Strahlenther Onkol. 2015 May;191(5):458-9. doi: 10.1007/s00066-015-0820-8. Strahlenther Onkol. 2015. PMID: 26120653 German. No abstract available.
-
Re: Duration of Androgen Suppression before Radiotherapy for Localized Prostate Cancer: Radiation Therapy Oncology Group Randomized Clinical Trial 9910.J Urol. 2016 Jan;195(1):96. doi: 10.1016/j.juro.2015.10.057. Epub 2015 Oct 23. J Urol. 2016. PMID: 26699964 No abstract available.
References
-
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma: Long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. - PubMed
-
- Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. - PubMed
-
- Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of Radiation Therapy Oncology Group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. - PubMed
-
- Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. - PubMed
-
- Lawton CA, DeSilvio M, Roach M, 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

