Risk of venous thromboembolism in patients with rheumatoid arthritis: initiating disease-modifying antirheumatic drugs
- PMID: 25534420
- PMCID: PMC4414700
- DOI: 10.1016/j.amjmed.2014.11.025
Risk of venous thromboembolism in patients with rheumatoid arthritis: initiating disease-modifying antirheumatic drugs
Abstract
Objectives: Recent research suggests that rheumatoid arthritis increases the risk of venous thromboembolism. This study compared the risk of venous thromboembolism in patients with newly diagnosed rheumatoid arthritis initiating a biologic disease-modifying antirheumatic drug (DMARD) with those initiating methotrexate or a nonbiologic DMARD.
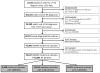
Methods: We conducted a population-based cohort study using US insurance claims data (2001-2012). Three mutually exclusive, hierarchical DMARD groups were used: (1) a biologic DMARD with and without nonbiologic DMARDs; (2) methotrexate without a biologic DMARD; or (3) nonbiologic DMARDs without a biologic DMARD or methotrexate. We calculated the incidence rates of venous thromboembolism. Cox proportional hazard models stratified by propensity score (PS) deciles after asymmetric PS trimming were used for 3 pairwise comparisons, controlling for potential confounders at baseline.
Results: We identified 29,481 patients with rheumatoid arthritis with 39,647 treatment episodes. From the pairwise comparison after asymmetric PS trimming, the incidence rate of hospitalization for venous thromboembolism per 1000 person-years was 5.5 in biologic DMARD initiators versus 4.4 in nonbiologic DMARD initiators and 4.8 in biologic DMARD initiators versus 3.5 in methotrexate initiators. The PS decile-stratified hazard ratio of venous thromboembolism associated with biologic DMARDs was 1.83 (95% confidence interval [CI], 0.91-3.66) versus nonbiologic DMARDs and 1.39 (95% CI, 0.73-2.63) versus methotrexate. The hazard ratio of venous thromboembolism in biologic DMARD initiators was the highest in the first 180 days versus nonbiologic DMARD initiators (2.48; 95% CI, 1.14-5.39) or methotrexate initiators (1.80; 95% CI, 0.90-3.62).
Conclusions: The absolute risk for venous thromboembolism was low in patients with newly diagnosed rheumatoid arthritis. Initiation of a biologic DMARD seems to be associated with an increased short-term risk of hospitalization for venous thromboembolism compared with initiation of a nonbiologic DMARD or methotrexate.
Keywords: Disease-modifying antirheumatic drugs; Rheumatoid arthritis; Venous thromboembolism.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Examining Time to Initiation of Biologic Disease-modifying Antirheumatic Drugs and Medication Adherence and Persistence Among Texas Medicaid Recipients With Rheumatoid Arthritis.Clin Ther. 2016 Mar;38(3):646-54. doi: 10.1016/j.clinthera.2016.01.022. Epub 2016 Feb 18. Clin Ther. 2016. PMID: 26899313
-
Factors associated with the initiation of biologic disease-modifying antirheumatic drugs in Texas Medicaid patients with rheumatoid arthritis.J Manag Care Spec Pharm. 2015 May;21(5):401-7. doi: 10.18553/jmcp.2015.21.5.401. J Manag Care Spec Pharm. 2015. PMID: 25943001 Free PMC article.
-
Biologic Disease-Modifying Antirheumatic Drugs and Risk of High-Grade Cervical Dysplasia and Cervical Cancer in Rheumatoid Arthritis: A Cohort Study.Arthritis Rheumatol. 2016 Sep;68(9):2106-13. doi: 10.1002/art.39689. Arthritis Rheumatol. 2016. PMID: 27015113 Free PMC article.
-
Efficacy and safety of methotrexate in combination with other non-biologic disease-modifying antirheumatic drugs (DMARDs) in treatment of rheumatoid arthritis.Bull Hosp Jt Dis (2013). 2013;71 Suppl 1:S20-8. Bull Hosp Jt Dis (2013). 2013. PMID: 24219037 Review.
-
Update on methotrexate as the anchor drug for rheumatoid arthritis.Bull Hosp Jt Dis (2013). 2013;71 Suppl 1:S9-19. Bull Hosp Jt Dis (2013). 2013. PMID: 24219036 Review.
Cited by
-
The association between autoantibodies and risk for venous thromboembolic events among patients with rheumatoid arthritis.Rheumatology (Oxford). 2023 Jun 1;62(6):2106-2112. doi: 10.1093/rheumatology/keac601. Rheumatology (Oxford). 2023. PMID: 36255271 Free PMC article.
-
Increased risk of cardiovascular events under the treatments with Janus kinase inhibitors versus biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a retrospective longitudinal population-based study using the Japanese health insurance database.RMD Open. 2024 Jun 17;10(2):e003885. doi: 10.1136/rmdopen-2023-003885. RMD Open. 2024. PMID: 38886005 Free PMC article.
-
Drug safety and immunogenicity of tumour necrosis factor inhibitors: the story so far.Rheumatology (Oxford). 2018 Nov 1;57(11):1896-1907. doi: 10.1093/rheumatology/kex434. Rheumatology (Oxford). 2018. PMID: 29325166 Free PMC article. Review.
-
Pulmonary embolism and infarction with a paradoxical thrombus visualised in both atria.BMJ Case Rep. 2018 May 30;2018:bcr2018225195. doi: 10.1136/bcr-2018-225195. BMJ Case Rep. 2018. PMID: 29848540 Free PMC article.
-
Tofacitinib in the treatment of Indian patients with rheumatoid arthritis: A post hoc analysis of efficacy and safety in Phase 3 and long-term extension studies over 7 years.Int J Rheum Dis. 2020 Jul;23(7):882-897. doi: 10.1111/1756-185X.13853. Epub 2020 Jun 1. Int J Rheum Dis. 2020. PMID: 32478474 Free PMC article.
References
-
- Choi HK, Rho YH, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis. 2012 [Epub ahead of print] - PubMed
-
- Holmqvist M, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA. 2012;308:1350–6. - PubMed
-
- Zöller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379:244–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

