Protection against Klebsiella pneumoniae using lithium chloride in an intragastric infection model
- PMID: 25534739
- PMCID: PMC4325792
- DOI: 10.1128/AAC.04261-14
Protection against Klebsiella pneumoniae using lithium chloride in an intragastric infection model
Abstract
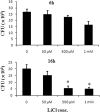
Intragastric Klebsiella pneumoniae infections of mice can cause liver abscesses, necrosis of liver tissues, and bacteremia. Lithium chloride, a widely prescribed drug for bipolar mood disorder, has been reported to possess anti-inflammatory properties. Using an intragastric infection model, the effects of LiCl on K. pneumoniae infections were examined. Providing mice with drinking water containing LiCl immediately after infection protected them from K. pneumoniae-induced death and liver injuries, such as necrosis of liver tissues, as well as increasing blood levels of aspartate aminotransferase and alanine aminotransferase, in a dose-dependent manner. LiCl administered as late as 24 h postinfection still provided protection. Monitoring of the LiCl concentrations in the sera of K. pneumoniae-infected mice showed that approximately 0.33 mM LiCl was the most effective dose for protecting mice against infections, which is lower than the clinically toxic dose of LiCl. Surveys of bacterial counts and cytokine expression levels in LiCl-treated mice revealed that both were effectively inhibited in blood and liver tissues. Using in vitro assays, we found that LiCl (5 μM to 1 mM) did not directly interfere with the growth of K. pneumoniae but made K. pneumoniae cells lose the mucoid phenotype and become more susceptible to macrophage killing. Furthermore, low doses of LiCl also partially enhanced the bactericidal activity of macrophages. Taken together, these data suggest that LiCl is an alternative therapeutic agent for K. pneumoniae-induced liver infections.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

