Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion
- PMID: 25535254
- PMCID: PMC4328644
- DOI: 10.15252/emmm.201404137
Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion
Abstract
Mycobacterium tuberculosis (MTB) remains a major challenge to global health made worse by the spread of multidrug resistance. We therefore examined whether stimulating intracellular killing of mycobacteria through pharmacological enhancement of macroautophagy might provide a novel therapeutic strategy. Despite the resistance of MTB to killing by basal autophagy, cell-based screening of FDA-approved drugs revealed two anticonvulsants, carbamazepine and valproic acid, that were able to stimulate autophagic killing of intracellular M. tuberculosis within primary human macrophages at concentrations achievable in humans. Using a zebrafish model, we show that carbamazepine can stimulate autophagy in vivo and enhance clearance of M. marinum, while in mice infected with a highly virulent multidrug-resistant MTB strain, carbamazepine treatment reduced bacterial burden, improved lung pathology and stimulated adaptive immunity. We show that carbamazepine induces antimicrobial autophagy through a novel, evolutionarily conserved, mTOR-independent pathway controlled by cellular depletion of myo-inositol. While strain-specific differences in susceptibility to in vivo carbamazepine treatment may exist, autophagy enhancement by repurposed drugs provides an easily implementable potential therapy for the treatment of multidrug-resistant mycobacterial infection.
Keywords: autophagy; multidrug‐resistant; myo‐inositol; tuberculosis.
© 2014 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
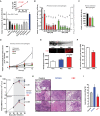
Screening drugs for effects on survival of intracellular mycobacteria in macrophages. RAW 264.7 cells were infected with a luminescent strain of M. bovis BCG (BCG-lux) for 1 h, washed and treated for 24 h with vehicle alone (white), known autophagy enhancers (interferon-γ (IFNγ), 200 ng/ml and rapamycin, 200 nM; black), known mTOR-independent autophagy inhibitor (forskolin; 24 μM; blue), carbamazepine (CBZ, 50 μM; red), valproic acid (VPA, 3 mM; green) and other examples of hits from a large screen of compounds enhancing intracellular killing of mycobacteria (lithium, 10 mM; rilmenidine, 1 μM; clonidine, 1 μM; calpeptin, 50 μM; grey). P-values, unpaired Student's t-test (n ≥ 6) (compared to vehicle alone): IFNγ 0.03; rapamycin 0.003, CBZ 0.001, VPA 0.001, lithium 0.001, rilmenidine 0.001; clonidine 0.02; calpeptin 0.01. Inset: Correlation between measurements of colony-forming units (CFU) and luminescence (RLU) for cultures of M. bovis BCG-lux as previously described (Kampmann et al, 2000).
Effects on intracellular survival of M. bovis BCG-lux of treatment with varying concentrations of CBZ (red) or VPA (green). P-values, unpaired Student's t-test (n = 6) (compared to vehicle alone) for CBZ 30 μM: 9 × 10−7, 40 μM: 1.9 × 10−9, 50 μM: 1.3 × 10−9, 100 μM: 1.6 × 10−8, 200 μM: 2.7 × 10−9, VPA 0.5 mM: 6.9 × 10−8, 1 mM: 1.6 × 10−9, 3 mM: 4.3 × 10−10, 4 mM: 4.2 × 10−9, 10 mM: 1.1 × 10−9, 20 mM: 4.2 × 10−10. These compounds had no effect on cell-free mycobacterial viability (grey circles).
Anticonvulsants enhance intracellular killing of mycobacteria within human alveolar macrophages. Alveolar macrophages, obtained from the broncho-alveolar lavage fluid of three individuals, were infected with M. bovis BCG-lux and then treated with carbamazepine (CBZ, 50 μM; red), valproic acid (VPA, 3 mM; green) or vehicle alone (Con; black). Viable intracellular mycobacteria were determined after 24 h of treatment. Unpaired Student's t-test (n = 3) (compared to vehicle alone): CBZ 0.011; VPA 0.0009.
Enhanced killing of intracellular M. tuberculosis (H37Rv) within primary human macrophages by treatment with CBZ (50 μM; red), VPA (3 mM; green) and rapamycin (200 nM; black), compared to control (vehicle alone; white). Unpaired Student's t-test (n = 6) (compared to vehicle alone): rapamycin 48 h 0.00002, rapamycin 72 h 0.00005, CBZ 48 h 0.00021, CBZ 72 h 0.00022, VPA 48 h 0.0002, VPA 72 h 0.00049.
In vivo induction of autophagy by CBZ (50 μM; red) and rapamycin (RAP; 1 μM; blue) compared to vehicle control (white) monitored in zebrafish expressing fluorescent ATG8 co-treated with chloroquine to delay degradation of autophagosomes.
Wild-type zebrafish were injected with a red fluorescently tagged M. marinum strain M into the yolk sac circulation valley at 28 hpf. Larvae were imaged at 120 hpf by confocal microscopy and the total mycobacteria-associated fluorescence quantified using Volocity® software. Data expressed as mean ± SEM (n ≥ 13 fish performed as 3 independent experiments). P-values, unpaired Student's t-test (compared to vehicle alone): 0.0035.
Mice infected via aerosol with a highly virulent clinical strain of multidrug-resistant M. tuberculosis CSU 87 were treated from day 20 post-infection with carbamazepine (CBZ, 50 μg/kg i.p. daily), rifampicin/isoniazid (RIF/INH) or vehicle control (n = 5 per time point per group). CBZ treatment for 30 days resulted in (G) significantly less viable bacteria detected in lung and spleen, (H) reduced inflammatory pulmonary infiltrates compared to RIF/INH-treated or control animals and (I) decreased lung lesion scores. Unpaired Student's t-test (n = 5) (compared to vehicle alone): RIF/INH 0.007; CBZ 0.037.

Levels of IL-8 (black) and TNFα (grey) released by M. bovis BCG-infected primary human macrophages were autophagy dependent, measured following siRNA knock-down of the critical autophagy protein, ATG12, or control siRNA (control). P-values, unpaired Student's t-test (n = 3) ATG12 siRNA vs siControl: TNFα 0.0006; IL-8 0.0012.
Carbamazepine increases pro-inflammatory cytokine secretion by mycobacteria-infected human macrophages. P-values, unpaired Student's t-test (n = 5) (compared to vehicle alone): CBZ TNFα 0.00048, IL-8 0.0017; Rap TNFα 0.001, IL-8 0.0089.
Analysis (by flow cytometry) of intracellular cytokines (IL-27, IL-12, TNFα) and MHC class II surface expression for dendritic cells (CD11c+) and macrophages (CD11b+) from lung, spleen and draining lymph nodes of mice that were uninfected and untreated (grey), uninfected and CBZ-treated (orange), infected with multidrug-resistant M. tuberculosis (MDR-TB) and untreated (black), MDR-TB infected and treated with rifampicin and isoniazid (RIF/INH; blue) or MDR-TB infected and treated with carbamazepine (CBZ, red).
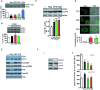
The anticonvulsants carbamazepine and valproic acid enhance autophagic clearance of cytosolic substrate. We monitored the clearance of the known autophagy substrate (A53T) α-synuclein in stable inducible PC12 cells by Western blot. The A53T α-synuclein transgene was induced with doxycycline for 48 h and then switched off (by antibiotic removal) before cells were treated with carbamazepine (CBZ, 50 μM; red), valproic acid (VPA, 3 mM; green), rapamycin (Rap, 200 nM; black), forskolin (FSK, 10 μM; blue) or vehicle alone (DMSO; white) for a further 24 h. P-values, paired Student's t-test (n = 3) (compared to vehicle alone): Rap 0.0011; CBZ 0.0017; VPA 0.02; FSK 0.005.
Anticonvulsants enhance autophagosome synthesis in primary human macrophages. In the presence of a saturating concentration of bafilomycin A1 (400 nM) which blocks autophagosome–lysosome fusion, levels of LC3-II reflect autophagosome production and were quantified in human macrophages (from a different healthy individual in 3 separate experiments) treated with rapamycin (Rap, 200 nM; black), carbamazepine (CBZ, 50 μM; red), valproic acid (VPA, 3 mM; green) or vehicle alone (DMSO; white). P-values, paired Student's t-test (n = 3) (compared to vehicle alone): CBZ 0.026.
Increases in LC3-II levels (assessed by Western blot) in primary human macrophages infected with M. bovis BCG after 4 h treatment with rapamycin (Rap; 200 nM), valproic acid (VPA, 3 mM) and carbamazepine (CBZ, 50 μM) compared to controls. P-values, paired Student's t-test (n = 3) (compared to vehicle alone): Rap 0.02; VPA 0.004; CBZ 0.003.
Treatment of M. bovis BCG-infected human macrophages with CBZ (50 μM) or VPA (3 mM) increased autophagosome number, assessed by confocal microscopy of cells stained with LC3-specific antibody (green) with quantification of the number of autophagosomes (defined as LC3 + vesicles ≥ 1 μm diameter) per cell shown below. P-values, unpaired Student's t-test (n = 3) (compared to vehicle alone): CBZ 0.001, VPA 0.003. Scale bar represents 10 μm.
In contrast to rapamycin (Rap), carbamazepine (CBZ) treatment of macrophages, while increasing LC3-II levels, does not alter mTOR-dependent signalling (monitored by changes in phosphorylation of S6 and p70S6 kinase).
Enhanced intracellular killing of mycobacteria by anticonvulsants is mediated through autophagy. SiRNA knock-down of ATG12 in primary human macrophages blocks autophagy leading to (i) reduced LC3-II levels and (ii) loss of CBZ- (50 μM) and VPA- (3 mM) induced enhancement of intracellular killing of M. bovis BCG. P-values, unpaired Student's t-test (n = 3) (compared to vehicle alone): Rap 0.002; VPA 0.001; CBZ 0.0001.
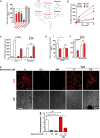
Effect on intracellular killing of luminescent mycobacteria in primary human macrophages of CBZ (50 μM), other dibenzazepines (opipramol, 100 ng/ml; lofepramine, 200 ng/ml; trimipramine, 50 ng/ml), mianserin (200 ng/ml) and the cardiac-specific SCN5A antagonist, flecainide (2 μg/ml). P-values, unpaired Student's t-test (n = ≥ 6) (compared to vehicle alone): CBZ 0.0001; opipramol 0.0005; lofepramine 0.0001; trimipramine 1 × 10−5.
CBZ inhibits Na+-dependent myo-inositol uptake by macrophages. Rates of [3H]myo-inositol accumulation within cells were determined in the presence (squares) or absence (circles) of extracellular Na+ during treatment with CBZ (50 μM; red) or vehicle alone (white). P-values, unpaired Student's t-test (n = 6) (compared to vehicle alone): CBZ 9.2 × 10−8.
Sodium-dependent myo-inositol uptake (at 5 min) by macrophages is reduced by siRNA knock-down of the Na+–inositol co-transporter SLC5A3 (SMIT-1) under which conditions co-treatment with CBZ (50 μM; red) caused no additional inhibition of inositol uptake.
Effect on intracellular killing of luminescent mycobacteria in primary human macrophages of (i) siRNA knock-down of the Na+–inositol co-transporter SLC5A3 (SMIT-1) (P-values, unpaired Student's t-test (n = 3) siSLC5A3 compared to siControl: 0.009; siControl + CBZ compared to vehicle alone: 0.0067) and (ii) incubation with excess extracellular myo-inositol with (red) or without (white) treatment with CBZ at 50 μM (n = 3).
Effect of depleting and adding excess myo-inositol on LC3-II mCherry autophagosome formation in RAW 264.7 macrophages. Myo-inositol depletion increases autophagosome formation (white), with no significant additional change with CBZ treatment (red). Excess myo-inositol further reduces autophagosome number (grey). P-values, unpaired Student's t-test (n = 3) (compared to myo-inositol-free): 0.2 μM 0.013, 200 μM 0.01; 0.2 μM compared 0.2 μM + CBZ: 0.023. Scale bar represents 40 μm.

Phospholipase C and IP3 receptor regulate autophagic killing of mycobacteria in Dictyostelium. Wild-type (WT, black) and mutant Dictyostelium AX2 strains were grown axenically and incubated with M. abscessus-lux (Renna et al, 2011) at an MOI of 0.01:1. M. abscessus-lux with no Dictyostelium (white) were grown under identical conditions as a control. Viable mycobacteria were quantified by measuring luminescence at 2 h post-infection. Mutant strains tested: mipp1− (multiple inositol polyphosphate phosphatase null), I6KA− (inositol hexakisphosphate phosphate kinase null); plc− (phospholipase C null); IP3R− (inositol (1,4,5)-trisphosphate receptor null mutant iplA-). P-values, unpaired Student's t-test (n = 3) (compared to wild-type): PLC− 0.01; IP3R− 0.007.
Quantification by mass spectroscopy of levels of C18:0 PIP2 species in macrophages following treatment with CBZ (red) or vehicle control (white). SA- C18:0/C20:4. P-values, unpaired Student's t-test (n = 3) (compared to vehicle alone): SA 6.6 × 10−9, 20:3 1.6 × 10−6, 18:2 3.5 × 10−8, 18:1 3.2 × 10−4.
Levels of basal IP3 (1,4,5) in macrophages following treatment with CBZ (red), lithium (white) or vehicle alone (black) for 24 h (top) or 48 h (bottom). P-values, unpaired Student's t-test (n ≥ 3) (compared to vehicle alone): CBZ 24 h 0.00007; CBZ 24 h 0.0013; LiCl 24 h 0.00005; LiCl 48 h 0.04.
Resting levels of mitochondrial calcium (monitored by mt-Cameleon sensor expression) are reduced by CBZ treatment (red) compared to controls. (i) Representative calcium recordings of individual mitochondria from CBZ-treated (red) or control (black) cells, matched for similar fluorescence levels after ionomycin addition (Fiono; arrow) showing lower resting mitochondrial calcium levels after treatment with CBZ. (ii) Normalized mitochondrial calcium levels (F/Fiono) from cells treated with CBZ (red) or vehicle alone (white). P-values, unpaired Student's t-test (n ≥ 20) (compared to vehicle alone): 9.5 × 10−15.
CBZ treatment of macrophages results in (E) a decrease in cellular ATP and consequently (F) increased phosphorylation of AMP kinase (AMPK) and ULK1 (ULK). P-values, unpaired Student's t-test (n = 3) (compared to vehicle alone): 0.00003.
Model for mechanism of autophagy induction by CBZ. Inhibition of the Na+ channel SCN5A leads to reduced activity of the Na+–inositol co-transported SLC5A3 leading to a drop in cellular inositol levels. The subsequent reduction in PIP2 (4,5) leads to a fall in basal IP3 (1,4,5) and reduced mitochondrial Ca2+ levels. The resultant fall in cellular ATP activates AMP kinase and subsequently ULK1 to trigger autophagic disposal of mycobacterial killing.
Similar articles
-
FDA-Approved Amoxapine Effectively Promotes Macrophage Control of Mycobacteria by Inducing Autophagy.Microbiol Spectr. 2022 Oct 26;10(5):e0250922. doi: 10.1128/spectrum.02509-22. Epub 2022 Sep 21. Microbiol Spectr. 2022. PMID: 36129262 Free PMC article.
-
Host-directed therapy with amiodarone in preclinical models restricts mycobacterial infection and enhances autophagy.Microbiol Spectr. 2024 Aug 6;12(8):e0016724. doi: 10.1128/spectrum.00167-24. Epub 2024 Jun 25. Microbiol Spectr. 2024. PMID: 38916320 Free PMC article.
-
Macrophage targeted graphene oxide nanosystem synergize antibiotic killing and host immune defense for Tuberculosis Therapy.Pharmacol Res. 2024 Oct;208:107379. doi: 10.1016/j.phrs.2024.107379. Epub 2024 Aug 30. Pharmacol Res. 2024. PMID: 39218421
-
An Interplay Between Autophagy and Immunometabolism for Host Defense Against Mycobacterial Infection.Front Immunol. 2020 Nov 12;11:603951. doi: 10.3389/fimmu.2020.603951. eCollection 2020. Front Immunol. 2020. PMID: 33262773 Free PMC article. Review.
-
Nanomaterial-mediated host directed therapy of tuberculosis by manipulating macrophage autophagy.J Nanobiotechnology. 2024 Oct 8;22(1):608. doi: 10.1186/s12951-024-02875-w. J Nanobiotechnology. 2024. PMID: 39379986 Free PMC article. Review.
Cited by
-
New TB treatments hiding in plain sight.EMBO Mol Med. 2015 Feb;7(2):125-6. doi: 10.15252/emmm.201404815. EMBO Mol Med. 2015. PMID: 25535253 Free PMC article.
-
Zebrafish modeling of intestinal injury, bacterial exposures and medications defines epithelial in vivo responses relevant to human inflammatory bowel disease.Dis Model Mech. 2019 Aug 13;12(8):dmm037432. doi: 10.1242/dmm.037432. Dis Model Mech. 2019. PMID: 31337664 Free PMC article.
-
Glibenclamide Reduces Primary Human Monocyte Functions Against Tuberculosis Infection by Enhancing M2 Polarization.Front Immunol. 2018 Sep 19;9:2109. doi: 10.3389/fimmu.2018.02109. eCollection 2018. Front Immunol. 2018. PMID: 30283449 Free PMC article.
-
A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy.Cells. 2019 Jul 3;8(7):674. doi: 10.3390/cells8070674. Cells. 2019. PMID: 31277291 Free PMC article. Review.
-
The potential of dibenzazepine carboxamides in cancer therapy.Front Pharmacol. 2025 Mar 28;16:1564911. doi: 10.3389/fphar.2025.1564911. eCollection 2025. Front Pharmacol. 2025. PMID: 40223925 Free PMC article. Review.
References
-
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. - PubMed
-
- Carrithers LM, Hulseberg P, Sandor M, Carrithers MD. The human macrophage sodium channel NaV1.5 regulates mycobacteria processing through organelle polarization and localized calcium oscillations. FEMS Immunol Med Microbiol. 2011;63:319–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0701932/MRC_/Medical Research Council/United Kingdom
- MR/M004864/1/MRC_/Medical Research Council/United Kingdom
- G108/595/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/000C0415/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U105115237/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

