Adult-onset deficiency of acyl CoA:monoacylglycerol acyltransferase 2 protects mice from diet-induced obesity and glucose intolerance
- PMID: 25535286
- PMCID: PMC4306691
- DOI: 10.1194/jlr.M055228
Adult-onset deficiency of acyl CoA:monoacylglycerol acyltransferase 2 protects mice from diet-induced obesity and glucose intolerance
Abstract
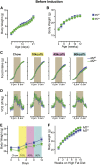
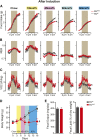
Acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 catalyzes triacylglycerol (TAG) synthesis, required in intestinal fat absorption. We previously demonstrated that mice without a functional MGAT2-coding gene (Mogat2(-/-)) exhibit increased energy expenditure and resistance to obesity induced by excess calories. One critical question raised is whether lacking MGAT2 during early development is required for the metabolic phenotypes in adult mice. In this study, we found that Mogat2(-/-) pups grew slower than wild-type littermates during the suckling period. To determine whether inactivating MGAT2 in adult mice is sufficient to confer resistance to diet-induced obesity, we generated mice with an inducible Mogat2-inactivating mutation. Mice with adult-onset MGAT2 deficiency (Mogat2(AKO)) exhibited a transient decrease in food intake like Mogat2(-/-) mice when fed a high-fat diet and a moderate increase in energy expenditure after acclimatization. They gained less weight than littermate controls, but the difference was smaller than that between wild-type and Mogat2(-/-) mice. The moderate reduction in weight gain was associated with reduced hepatic TAG and improved glucose tolerance. Similar protective effects were also observed in mice that had gained weight on a high-fat diet before inactivating MGAT2. These findings suggest that adult-onset MGAT2 deficiency mitigates metabolic disorders induced by high-fat feeding and that MGAT2 modulates early postnatal nutrition and may program metabolism later in life.
Keywords: coenzyme A; dietary fat; energy expenditure; fat absorption; metabolic programming; monoacylglycerol acyltransferase 2; triacylglycerol.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Coleman R. A., Lee D. P. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43: 134–176. - PubMed
-
- Yen C. L., Farese R. V., Jr 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. - PubMed
-
- Cao J., Hawkins E., Brozinick J., Liu X., Zhang H., Burn P., Shi Y. 2004. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J. Biol. Chem. 279: 18878–18886. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

