Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity
- PMID: 25535287
- PMCID: PMC4306695
- DOI: 10.1194/jlr.M055798
Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity
Abstract
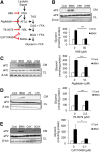
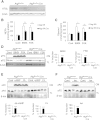
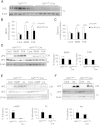
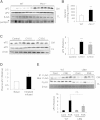
Adipocyte fatty acid binding protein 4, aP2, contributes to the pathogenesis of several common diseases including type 2 diabetes, atherosclerosis, fatty liver disease, asthma, and cancer. Although the biological functions of aP2 have classically been attributed to its intracellular action, recent studies demonstrated that aP2 acts as an adipokine to regulate systemic metabolism. However, the mechanism and regulation of aP2 secretion remain unknown. Here, we demonstrate a specific role for lipase activity in aP2 secretion from adipocytes in vitro and ex vivo. Our results show that chemical inhibition of lipase activity, genetic deficiency of adipose triglyceride lipase and, to a lesser extent, hormone-sensitive lipase blocked aP2 secretion from adipocytes. Increased lipolysis and lipid availability also contributed to aP2 release as determined in perilipin1-deficient adipose tissue explants ex vivo and upon treatment with lipids in vivo and in vitro. In addition, we identify a nonclassical route for aP2 secretion in exosome-like vesicles and show that aP2 is recruited to this pathway upon stimulation of lipolysis. Given the effect of circulating aP2 on glucose metabolism, these data support that targeting aP2 or the lipolysis-dependent secretory pathway may present novel mechanistic and translational opportunities in metabolic disease.
Keywords: adipokine; adipose triglyceride lipase; hormone; hormone-sensitive lipase; lipolysis; obesity.
Figures

References
-
- Kershaw E. E., Flier J. S. 2004. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89: 2548–2556. - PubMed
-
- Scherer P. E. 2006. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 55: 1537–1545. - PubMed
-
- Hertzel A. V., Bernlohr D. A. 2000. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol. Metab. 11: 175–180. - PubMed
-
- Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., Spiegelman B. M. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 274: 1377–1379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

