A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome
- PMID: 25535367
- PMCID: PMC4291642
- DOI: 10.1073/pnas.1421420112
A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome
Abstract
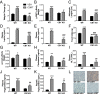
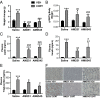
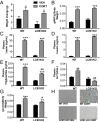
Glucocorticoids are known to promote the development of metabolic syndrome through the modulation of both feeding pathways and metabolic processes; however, the precise mechanisms of these effects are not well-understood. Recent evidence shows that glucocorticoids possess the ability to increase endocannabinoid signaling, which is known to regulate appetite, energy balance, and metabolic processes through both central and peripheral pathways. The aim of this study was to determine the role of endocannabinoid signaling in glucocorticoid-mediated obesity and metabolic syndrome. Using a mouse model of excess corticosterone exposure, we found that the ability of glucocorticoids to increase adiposity, weight gain, hormonal dysregulation, hepatic steatosis, and dyslipidemia was reduced or reversed in mice lacking the cannabinoid CB1 receptor as well as mice treated with the global CB1 receptor antagonist AM251. Similarly, a neutral, peripherally restricted CB1 receptor antagonist (AM6545) was able to attenuate the metabolic phenotype caused by chronic corticosterone, suggesting a peripheral mechanism for these effects. Biochemical analyses showed that chronic excess glucocorticoid exposure produced a significant increase in hepatic and circulating levels of the endocannabinoid anandamide, whereas no effect was observed in the hypothalamus. To test the role of the liver, specific and exclusive deletion of hepatic CB1 receptor resulted in a rescue of the dyslipidemic effects of glucocorticoid exposure, while not affecting the obesity phenotype or the elevations in insulin and leptin. Together, these data indicate that glucocorticoids recruit peripheral endocannabinoid signaling to promote metabolic dysregulation, with hepatic endocannabinoid signaling being especially important for changes in lipid metabolism.
Keywords: 2-AG; anandamide; corticosterone; liver; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rosenzweig JL, et al. Endocrine Society Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(10):3671–3689. - PubMed
-
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. - PubMed
-
- Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and adiposity: A meta-analysis of longitudinal studies. Obesity (Silver Spring) 2011;19(4):771–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

