Ginsenoside fractions regulate the action of monocytes and their differentiation into dendritic cells
- PMID: 25535474
- PMCID: PMC4268565
- DOI: 10.1016/j.jgr.2014.07.003
Ginsenoside fractions regulate the action of monocytes and their differentiation into dendritic cells
Abstract
Background: Panax ginseng (i.e., ginseng) root is extensively used in traditional oriental medicine. It is a modern pharmaceutical reagent for preventing various human diseases such as cancer. Ginsenosides-the major active components of ginseng-exhibit immunomodulatory effects. However, the mechanism and function underlying such effects are not fully elucidated, especially in human monocytes and dendritic cells (DCs).
Methods: We investigated the immunomodulatory effect of ginsenosides from Panax ginseng root on CD14(+) monocytes purified from human adult peripheral blood mononuclear cells (PBMCs) and on their differentiation into DCs that affect CD4(+) T cell activity.
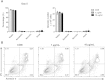
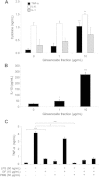
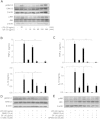
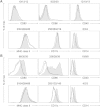
Results: After treatment with ginsenoside fractions, monocyte levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 increased through phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK), but not p38 mitogen-activated protein kinase (MAPK). After treatment with ginsenoside fractions, TNF-α production and phosphorylation of ERK1/2 and JNK decreased in lipopolysaccharide (LPS)-sensitized monocytes. We confirmed that DCs derived from CD14(+) monocytes in the presence of ginsenoside fractions (Gin-DCs) contained decreased levels of the costimulatory molecules CD80 and CD86. The expression of these costimulatory molecules decreased in LPS-treated DCs exposed to ginsenoside fractions, compared to their expression in LPS-treated DCs in the absence of ginsenoside fractions. Furthermore, LPS-treated Gin-DCs could not induce proliferation and interferon gamma (IFN-γ) production by CD4(+) T cells with the coculture of Gin-DCs with CD4+ T cells.
Conclusion: These results suggest that ginsenoside fractions from the ginseng root suppress cytokine production and maturation of LPS-treated DCs and downregulate CD4(+) T cells.
Keywords: CD14+ monocytes; CD4+ T cells; Panax ginseng; dendritic cells; ginsenosides.
Figures

References
-
- Kiefer D., Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–1542. - PubMed
-
- Lee T.K., Johnke R.M., Allison R.R., O'Brien K.F., Dobbs L.J., Jr. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. - PubMed
-
- Kang K.A., Kang J.H., Yang M.P. Ginseng total saponin enhances the phagocytic capacity of canine peripheral blood phagocytes in vitro. Am J Chin Med. 2008;36:329–341. - PubMed
-
- Fan Z.H., Isobe K., Kiuchi K., Nakashima I. Enhancement of nitric oxide production from activated macrophages by a purified form of ginsenoside (Rg1) Am J Chin Med. 1995;23:279–287. - PubMed
-
- Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

