Glucocorticoid regulation of lung development: lessons learned from conditional GR knockout mice
- PMID: 25535891
- PMCID: PMC5414758
- DOI: 10.1210/me.2014-1362
Glucocorticoid regulation of lung development: lessons learned from conditional GR knockout mice
Abstract
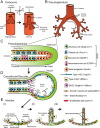
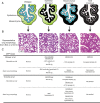
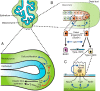
Glucocorticoid (GC) steroid hormones have well-characterized roles in the regulation of systemic homeostasis, yet less understood is their known role in utero to mature the developing respiratory system in preparation for birth. During late gestation, endogenously produced GCs thin the interstitial tissue of the lung, causing the vasculature and future airspaces to come into close alignment, allowing for efficient gas exchange at birth. More potent synthetic GCs are also used worldwide to reduce the severity of respiratory distress suffered by preterm infants; however, their clinical benefits are somewhat offset by potential detrimental long-term effects on health and development. Here, we review the recent literature studying both global and conditional gene-targeted respiratory mouse models of either GC deficiency or glucocorticoid receptor ablation. Although some discrepancies exist between these transgenic mouse strains, these models have revealed specific roles for GCs in particular tissue compartments of the developing lung and identify the mesenchyme as the critical site for glucocorticoid receptor-mediated lung maturation, particularly for the inhibition of cell proliferation and epithelial cell differentiation. Specific mesenchymal and epithelial cell-expressed gene targets that may potentially mediate the effect of GCs have also been identified in these studies and imply a GC-regulated system of cross talk between compartments during lung development. A better understanding of the specific roles of GCs in specific cell types and compartments of the fetal lung will allow the development of a new generation of selective GC ligands, enabling better therapeutic treatments with fewer side effects for lung immaturity at birth in preterm infants.
Figures

Similar articles
-
Mesenchymal glucocorticoid receptor regulates the development of multiple cell layers of the mouse lung.Am J Respir Cell Mol Biol. 2014 Feb;50(2):419-28. doi: 10.1165/rcmb.2013-0169OC. Am J Respir Cell Mol Biol. 2014. PMID: 24053134
-
Glucocorticoid metabolism in the developing lung: adrenal-like synthesis pathway.J Steroid Biochem Mol Biol. 2013 Nov;138:72-80. doi: 10.1016/j.jsbmb.2013.03.004. Epub 2013 Mar 26. J Steroid Biochem Mol Biol. 2013. PMID: 23537622 Review.
-
Glucocorticoid signalling drives reduced versican levels in the fetal mouse lung.J Mol Endocrinol. 2020 Apr;64(3):155-164. doi: 10.1530/JME-19-0235. J Mol Endocrinol. 2020. PMID: 31958317
-
Glucocorticoid Resistance: Interference between the Glucocorticoid Receptor and the MAPK Signalling Pathways.Int J Mol Sci. 2021 Sep 17;22(18):10049. doi: 10.3390/ijms221810049. Int J Mol Sci. 2021. PMID: 34576214 Free PMC article. Review.
-
Altered epithelial cell proportions in the fetal lung of glucocorticoid receptor null mice.Am J Respir Cell Mol Biol. 2004 May;30(5):613-9. doi: 10.1165/rcmb.2003-0236OC. Epub 2003 Oct 24. Am J Respir Cell Mol Biol. 2004. PMID: 14578211
Cited by
-
Creb1 regulates late stage mammalian lung development via respiratory epithelial and mesenchymal-independent mechanisms.Sci Rep. 2016 May 6;6:25569. doi: 10.1038/srep25569. Sci Rep. 2016. PMID: 27150575 Free PMC article.
-
Antenatal dexamethasone treatment transiently alters diastolic function in the mouse fetal heart.J Endocrinol. 2019 Jun 1;241(3):279-292. doi: 10.1530/JOE-18-0666. J Endocrinol. 2019. PMID: 31013474 Free PMC article.
-
Efficacy and safety of antenatal steroids.Am J Physiol Regul Integr Comp Physiol. 2018 Oct 1;315(4):R825-R839. doi: 10.1152/ajpregu.00193.2017. Epub 2018 Apr 11. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29641233 Free PMC article. Review.
-
The Influence of Light Exposure in Ambiance during Pregnancy in Maternal and Fetal Outcomes: An Experimental Study.Rev Bras Ginecol Obstet. 2019 Jan;41(1):24-30. doi: 10.1055/s-0038-1675610. Epub 2018 Nov 14. Rev Bras Ginecol Obstet. 2019. PMID: 30428491 Free PMC article.
-
Prenatal inflammation enhances antenatal corticosteroid-induced fetal lung maturation.JCI Insight. 2020 Dec 17;5(24):e139452. doi: 10.1172/jci.insight.139452. JCI Insight. 2020. PMID: 33328385 Free PMC article.
References
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525. - PubMed
-
- Cole TJ, Blendy JA, Monaghan AP, et al. . Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

