Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour
- PMID: 25536025
- PMCID: PMC4495958
- DOI: 10.1038/ncomms6759
Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour
Abstract
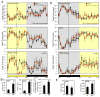
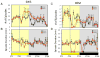
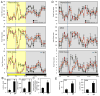
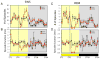
Synthetic drug-like molecules that directly modulate the activity of key clock proteins offer the potential to directly modulate the endogenous circadian rhythm and treat diseases associated with clock dysfunction. Here we demonstrate that synthetic ligands targeting a key component of the mammalian clock, the nuclear receptors REV-ERBα and β, regulate sleep architecture and emotional behaviour in mice. REV-ERB agonists induce wakefulness and reduce REM and slow-wave sleep. Interestingly, REV-ERB agonists also reduce anxiety-like behaviour. These data are consistent with increased anxiety-like behaviour of REV-ERBβ-null mice, in which REV-ERB agonists have no effect. These results indicate that pharmacological targeting of REV-ERB may lead to the development of novel therapeutics to treat sleep disorders and anxiety.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



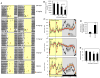
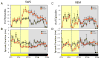
Comment in
-
Sleep-related disorders: Expanding the applications of clock-gene modulation.Nat Rev Drug Discov. 2015 Feb;14(2):92. doi: 10.1038/nrd4541. Nat Rev Drug Discov. 2015. PMID: 25633790 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

