Extent of non-publication in cohorts of studies approved by research ethics committees or included in trial registries
- PMID: 25536072
- PMCID: PMC4275183
- DOI: 10.1371/journal.pone.0114023
Extent of non-publication in cohorts of studies approved by research ethics committees or included in trial registries
Abstract
Background: The synthesis of published research in systematic reviews is essential when providing evidence to inform clinical and health policy decision-making. However, the validity of systematic reviews is threatened if journal publications represent a biased selection of all studies that have been conducted (dissemination bias). To investigate the extent of dissemination bias we conducted a systematic review that determined the proportion of studies published as peer-reviewed journal articles and investigated factors associated with full publication in cohorts of studies (i) approved by research ethics committees (RECs) or (ii) included in trial registries.
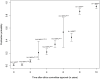
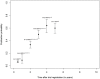
Methods and findings: Four bibliographic databases were searched for methodological research projects (MRPs) without limitations for publication year, language or study location. The searches were supplemented by handsearching the references of included MRPs. We estimated the proportion of studies published using prediction intervals (PI) and a random effects meta-analysis. Pooled odds ratios (OR) were used to express associations between study characteristics and journal publication. Seventeen MRPs (23 publications) evaluated cohorts of studies approved by RECs; the proportion of published studies had a PI between 22% and 72% and the weighted pooled proportion when combining estimates would be 46.2% (95% CI 40.2%-52.4%, I2 = 94.4%). Twenty-two MRPs (22 publications) evaluated cohorts of studies included in trial registries; the PI of the proportion published ranged from 13% to 90% and the weighted pooled proportion would be 54.2% (95% CI 42.0%-65.9%, I2 = 98.9%). REC-approved studies with statistically significant results (compared with those without statistically significant results) were more likely to be published (pooled OR 2.8; 95% CI 2.2-3.5). Phase-III trials were also more likely to be published than phase II trials (pooled OR 2.0; 95% CI 1.6-2.5). The probability of publication within two years after study completion ranged from 7% to 30%.
Conclusions: A substantial part of the studies approved by RECs or included in trial registries remains unpublished. Due to the large heterogeneity a prediction of the publication probability for a future study is very uncertain. Non-publication of research is not a random process, e.g., it is associated with the direction of study findings. Our findings suggest that the dissemination of research findings is biased.
Conflict of interest statement
Figures



References
-
- Simes RJ (1986) Publication bias: the case for an international registry of clinical trials. J Clin Oncol 4:1529–1541. - PubMed
-
- Dickersin K (1990) The existence of publication bias and risk factors for its occurrence. JAMA 263:1385–1389. - PubMed
-
- Ioannidis JP (1998) Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA 279:281–286. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

