A randomized, placebo controlled pilot trial of botulinum toxin for paratonic rigidity in people with advanced cognitive impairment
- PMID: 25536218
- PMCID: PMC4275182
- DOI: 10.1371/journal.pone.0114733
A randomized, placebo controlled pilot trial of botulinum toxin for paratonic rigidity in people with advanced cognitive impairment
Abstract
Objective: Evaluate safety and efficacy of Incobotulinumtoxin A in elderly patients with dementia and paratonia.
Setting: University-affiliated hospital, spasticity management Clinic.
Participants: Ten subjects were enrolled.
Inclusion criteria: 1) severe cognitive impairment 2) diagnosis of Alzheimer's disease, vascular dementia, or frontotemporal dementia, and 3) score >3 on the paratonic assessment instrument, with posture in an arm(s) interfering with provision of care.
Exclusion criteria: 1) alternate etiologies for increased tone and 2) injection with botulinum toxin within the 6 months preceding the study.
Design: Single center, randomized, double blind, placebo-controlled, crossover trial with two treatment cycles of 16 weeks. Assessments occurred at 2, 6, 12 and16 weeks following injections. Subjects received up to 300 U of Incobotulinumtoxin A in arm(s).
Primary and secondary outcome measures: Primary outcome measure was the modified caregiver burden scale (mCBS); exploratory secondary outcome measures were also performed. Analysis of variance and mixed modeling techniques were used to evaluate treatment effects.
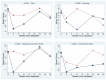
Results: Incobotulinumtoxin A treatment produced significant improvement in mCBS total score -1.11 (-2.04 to -0.18) (Treatment effect and 95% CI), dressing sub-score -0.36 (-0.59 to 0.12), and cleaning under the left and right armpits sub-score -0.5 (-0.96 to -0.04), -0.41 (-0.79 to -0.04) respectively. PROM in the left and right elbow increased by 27.67 degrees (13.32-42.02) and 22.07 degrees (9.76-34.39) respectively. PROM in the left and right shoulder increased by 11.92 degrees (5.46-18.38) and 8.58 degrees (3.73-13.43) respectively. No significant treatment effect was found for GAS, VAS and PAINAD scales or change in time to perform care. No adverse drug reactions occurred.
Conclusions: Administration of Incobotulinumtoxin A in elderly people with advanced dementia and paratonia may be an efficacious and safe treatment to increase range of motion and reduce functional burden. Further studies are needed to confirm results.
Trial registration: ClinicalTrials.Gov NCT02212119.
Conflict of interest statement
Figures
References
-
- Dupre E (1910) Debilite mentale and debilite motrice associees. Rev Neurol 20:54–56.
-
- Hobbelen JS, Koopmans RT, Verhey FR, Habraken KM, de Bie RA (2008) Diagnosing paratonia in the demented elderly: reliability and validity of the Paratonia Assessment Instrument (PAI). Int Psychogeriatr 20:840–852. - PubMed
-
- Franssen EH, Kluger A, Torossian CL, Reisberg B (1993) The neurologic syndrome of severe Alzheimer’s disease. Relationship to functional decline. Arch Neurol 50:1029–1039. - PubMed
-
- Souren LE, Franssen EH, Reisberg B (1997) Neuromotor changes in Alzheimer’s disease: implications for patient care. J Geriatr Psychiatry Neurol 10:93–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical