Synchrotron X-ray diffraction to detect glass or ice formation in the vitrified bovine cumulus-oocyte complexes and morulae
- PMID: 25536435
- PMCID: PMC4275205
- DOI: 10.1371/journal.pone.0114801
Synchrotron X-ray diffraction to detect glass or ice formation in the vitrified bovine cumulus-oocyte complexes and morulae
Abstract
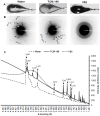
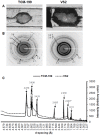
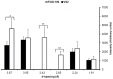
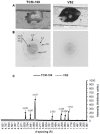
Vitrification of bovine cumulus-oocyte complexes (COCs) is not as successful as bovine embryos, due to oocyte's complex structure and chilling sensitivity. Synchrotron X-ray diffraction (SXRD), a powerful method to study crystal structure and phase changes, was used to detect the glass or ice formation in water, tissue culture medium (TCM)-199, vitrification solution 2 (VS2), and vitrified bovine COCs and morulae. Data revealed Debye's rings and peaks associated with the hexagonal ice crystals at 3.897, 3.635, 3.427, 2.610, 2.241, 1.912 and 1.878 Å in both water and TCM-199, whereas VS2 showed amorphous (glassy) appearance, at 102K (-171°C). An additional peak of sodium phosphate monobasic hydrate (NaH2PO4.H2O) crystals was observed at 2.064 Å in TCM-199 only. All ice and NaH2PO4.H2O peaks were detected in the non-vitrified (control) and vitrified COCs, except two ice peaks (3.145 and 2.655 Å) were absent in the vitrified COCs. The intensities of majority of ice peaks did not differ between the non-vitrified and vitrified COCs. The non-vitrified bovine morulae in TCM-199 demonstrated all ice- and NaH2PO4.H2O-associated Debye's rings and peaks, found in TCM-199 alone. There was no Debye's ring present in the vitrified morulae. In conclusion, SXRD is a powerful method to confirm the vitrifiability of a solution and to detect the glass or ice formation in vitrified cells and tissues. The vitrified bovine COCs exhibited the hexagonal ice crystals instead of glass formation whereas the bovine morulae underwent a typical vitrification.
Conflict of interest statement
Figures


Similar articles
-
Vitrification of immature bovine cumulus-oocyte complexes: effects of cryoprotectants, the vitrification procedure and warming time on cleavage and embryo development.Reprod Biol Endocrinol. 2012 Sep 6;10:73. doi: 10.1186/1477-7827-10-73. Reprod Biol Endocrinol. 2012. PMID: 22954348 Free PMC article.
-
Factors affecting nuclear maturation, cleavage and embryo development of vitrified bovine cumulus-oocyte complexes.Theriogenology. 2011 Mar 1;75(4):602-9. doi: 10.1016/j.theriogenology.2010.09.027. Epub 2010 Dec 28. Theriogenology. 2011. PMID: 21190729
-
Bovine oocyte vitrification using the Cryotop method: effect of cumulus cells and vitrification protocol on survival and subsequent development.Cryobiology. 2010 Aug;61(1):66-72. doi: 10.1016/j.cryobiol.2010.05.002. Epub 2010 May 25. Cryobiology. 2010. PMID: 20510225
-
Vitrification with DAP 213 and cryotop of ex situ and in situ feline cumulus-oocyte complexes.Reprod Domest Anim. 2012 Dec;47(6):1003-8. doi: 10.1111/j.1439-0531.2012.02006.x. Epub 2012 Mar 3. Reprod Domest Anim. 2012. PMID: 22384830
-
Effect of vitrification on meiotic maturation and expression of genes in immature goat cumulus oocyte complexes.Cryobiology. 2012 Jun;64(3):176-84. doi: 10.1016/j.cryobiol.2012.01.005. Epub 2012 Jan 18. Cryobiology. 2012. PMID: 22280956
Cited by
-
Ice formation and its elimination in cryopreservation of oocytes.Res Sq [Preprint]. 2024 May 23:rs.3.rs-4144933. doi: 10.21203/rs.3.rs-4144933/v1. Res Sq. 2024. Update in: Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. PMID: 38826214 Free PMC article. Updated. Preprint.
-
Characterization of Laser Gold Nanowarming: A Platform for Millimeter-Scale Cryopreservation.Langmuir. 2019 Jun 11;35(23):7364-7375. doi: 10.1021/acs.langmuir.8b03011. Epub 2018 Oct 25. Langmuir. 2019. PMID: 30299961 Free PMC article.
-
Conduction Cooling and Plasmonic Heating Dramatically Increase Droplet Vitrification Volumes for Cell Cryopreservation.Adv Sci (Weinh). 2021 Apr 10;8(11):2004605. doi: 10.1002/advs.202004605. eCollection 2021 Jun. Adv Sci (Weinh). 2021. PMID: 34141523 Free PMC article.
-
Transcriptomic difference in bovine blastocysts following vitrification and slow freezing at morula stage.PLoS One. 2017 Nov 2;12(11):e0187268. doi: 10.1371/journal.pone.0187268. eCollection 2017. PLoS One. 2017. PMID: 29095916 Free PMC article.
-
Ice formation and its elimination in cryopreservation of oocytes.Sci Rep. 2024 Aug 13;14(1):18809. doi: 10.1038/s41598-024-69528-8. Sci Rep. 2024. PMID: 39138273 Free PMC article.
References
-
- Pereira RM, Marques CC (2008) Animal oocyte and embryo cryopreservation. Cell Tissue Banking 9:267–277. - PubMed
-
- Hunter JE, Fuller BJ, Bernard A, Jackson A, Shaw RW (1995) Vitrification of human oocytes following minimal exposure to cryoprotectants; initial studies on fertilization and embryonic development. Hum Reprod 10:1184–1188. - PubMed
-
- Albarracin JL, Morato R, Izquierdo D, Mogas T (2005) Vitrification of calf oocytes: effects of maturation stage and prematuration treatment on the nuclear cytoskeletal components of oocytes and their subsequent development. Mol Reprod Dev 72:239–249. - PubMed
-
- Martino A, Songsasen N, Leibo SP (1996) Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod 54:1059–1069. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

