Macrophages contribute to the cyclic activation of adult hair follicle stem cells
- PMID: 25536657
- PMCID: PMC4275176
- DOI: 10.1371/journal.pbio.1002002
Macrophages contribute to the cyclic activation of adult hair follicle stem cells
Abstract
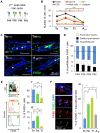
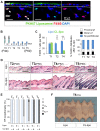
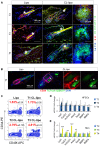
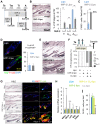
Skin epithelial stem cells operate within a complex signaling milieu that orchestrates their lifetime regenerative properties. The question of whether and how immune cells impact on these stem cells within their niche is not well understood. Here we show that skin-resident macrophages decrease in number because of apoptosis before the onset of epithelial hair follicle stem cell activation during the murine hair cycle. This process is linked to distinct gene expression, including Wnt transcription. Interestingly, by mimicking this event through the selective induction of macrophage apoptosis in early telogen, we identify a novel involvement of macrophages in stem cell activation in vivo. Importantly, the macrophage-specific pharmacological inhibition of Wnt production delays hair follicle growth. Thus, perifollicular macrophages contribute to the activation of skin epithelial stem cells as a novel, additional cue that regulates their regenerative activity. This finding may have translational implications for skin repair, inflammatory skin diseases and cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

