Leptin's effect on taste bud calcium responses and transmitter secretion
- PMID: 25537017
- PMCID: PMC4398048
- DOI: 10.1093/chemse/bju066
Leptin's effect on taste bud calcium responses and transmitter secretion
Abstract
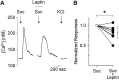
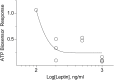
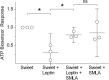
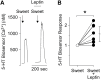
Leptin, a peptide hormone released by adipose tissue, acts on the hypothalamus to control cravings and appetite. Leptin also acts to decrease taste responses to sweet substances, though there is little detailed information regarding where leptin acts in the taste transduction cascade. The present study examined the effects of leptin on sweet-evoked responses and neuro transmitter release from isolated taste buds. Our results indicate that leptin moderately decreased sweet-evoked calcium mobilization in isolated mouse taste buds. We also employed Chinese hamster ovary biosensor cells to examine taste transmitter release from isolated taste buds. Leptin reduced ATP and increased serotonin release in response to sweet stimulation. However, leptin has no effect on bitter-evoked transmitter release, further showing that the action of leptin is sweet specific. Our results support those of previous studies, which state that leptin acts on taste tissue via the leptin receptor, most likely on Type II (Receptor) cells, but also possibly on Type III (Presynaptic) cells.
Keywords: ATP; leptin; serotonin; taste bud.
© The Author 2014. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Taste bud leptin: sweet dampened at initiation site.Chem Senses. 2015 May;40(4):213-5. doi: 10.1093/chemse/bjv004. Epub 2015 Mar 4. Chem Senses. 2015. PMID: 25740303 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

