Identification of a Dutch founder mutation in MUSK causing fetal akinesia deformation sequence
- PMID: 25537362
- PMCID: PMC4538208
- DOI: 10.1038/ejhg.2014.273
Identification of a Dutch founder mutation in MUSK causing fetal akinesia deformation sequence
Abstract
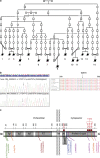
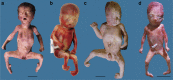
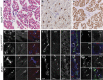
Fetal akinesia deformation sequence (FADS) refers to a clinically and genetically heterogeneous group of disorders with congenital malformations related to impaired fetal movement. FADS can result from mutations in CHRNG, CHRNA1, CHRND, DOK7 and RAPSN; however, these genes only account for a minority of cases. Here we identify MUSK as a novel cause of lethal FADS. Fourteen affected fetuses from a Dutch genetic isolate were traced back to common ancestors 11 generations ago. Homozygosity mapping in two fetuses revealed MUSK as a candidate gene. All tested cases carried an identical homozygous variant c.1724T>C; p.(Ile575Thr) in the intracellular domain of MUSK. The carrier frequency in the genetic isolate was 8%, exclusively found in heterozygous carriers. Consistent with the established role of MUSK as a tyrosine kinase that orchestrates neuromuscular synaptogenesis, the fetal myopathy was accompanied by impaired acetylcholine receptor clustering and reduced tyrosine kinase activity at motor nerve endings. A functional assay in myocytes derived from human fetuses confirmed that the variant blocks MUSK-dependent motor endplate formation. Taken together, the results strongly support a causal role of this founder mutation in MUSK, further expanding the gene set associated with FADS and offering new opportunities for prenatal genetic testing.
Figures

References
-
- Hall JG: Pena-Shokeir phenotype (fetal akinesia deformation sequence) revisited. Birth Defects Res A Clin Mol Teratol 2009; 85: 677–694. - PubMed
-
- Pena SD, Shokeir MH: Syndrome of camptodactyly, multiple ankyloses, facial anomalies, and pulmonary hypoplasia: a lethal condition. J Pediatr 1974; 85: 373–375. - PubMed
-
- Ravenscroft G, Sollis E, Charles AK, North KN, Baynam G, Laing NG: Fetal akinesia: review of the genetics of the neuromuscular causes. J Med Genet 2011; 48: 793–801. - PubMed
-
- Vogt J, Morgan NV, Rehal P et al: CHRNG genotype-phenotype correlations in the multiple pterygium syndromes. J Med Genet 2012; 49: 21–26. - PubMed
-
- DeChiara TM, Bowen DC, Valenzuela DM et al: The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996; 85: 501–512. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

