Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing's disease: results from an open-ended, open-label extension trial
- PMID: 25537481
- PMCID: PMC4560758
- DOI: 10.1007/s11102-014-0618-1
Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing's disease: results from an open-ended, open-label extension trial
Abstract
Purpose: Report the efficacy and safety of pasireotide sc in patients with Cushing's disease during an open-ended, open-label extension to a randomized, double-blind, 12-month, Phase III study.
Methods: 162 patients entered the core study. 58 patients who had mean UFC ≤ ULN at month 12 or were benefiting clinically from pasireotide entered the extension. Patients received the same dose of pasireotide as at the end of the core study (300-1,200 μg bid). Dose titration was permitted according to efficacy or drug-related adverse events.
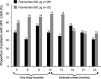
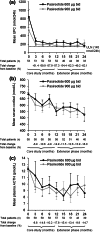
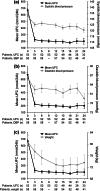
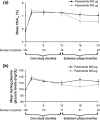
Results: 40 patients completed 24 months' treatment. Of the patients who entered the extension, 50.0% (29/58) and 34.5% (20/58) had controlled UFC (UFC ≤ ULN) at months 12 and 24, respectively. The mean percentage decrease in UFC was 57.3% (95% CI 40.7-73.9; n = 52) and 62.1% (50.8-73.5; n = 33) after 12 and 24 months' treatment, respectively. Improvements in clinical signs of Cushing's disease were sustained up to month 24. The most frequent drug-related adverse events in patients who received ≥1 dose of pasireotide (n = 162) from core baseline until the 24-month cut-off were diarrhea (55.6%), nausea (48.1%), hyperglycemia (38.9%), and cholelithiasis (31.5%). No new safety issues were identified during the extension.
Conclusions: Reductions in mean UFC and improvements in clinical signs of Cushing's disease were maintained over 24 months of pasireotide treatment. The safety profile of pasireotide is typical for a somatostatin analogue, except for the frequency and degree of hyperglycemia; patients should be monitored for changes in glucose homeostasis. Pasireotide represents the first approved pituitary-targeted treatment for patients with Cushing's disease.
Trial registration: ClinicalTrials.gov NCT00434148.
Figures
References
-
- Biller BMK, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93:2454–2462. doi: 10.1210/jc.2007-2734. - DOI - PMC - PubMed
-
- Data from CDC statistics and NHANES III (2005–2006). 2011. Available at: http://www.cdc.gov/nchs/nhanes.htm
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

