Safety and effectiveness of dipeptidyl peptidase-4 inhibitors versus intermediate-acting insulin or placebo for patients with type 2 diabetes failing two oral antihyperglycaemic agents: a systematic review and network meta-analysis
- PMID: 25537781
- PMCID: PMC4275675
- DOI: 10.1136/bmjopen-2014-005752
Safety and effectiveness of dipeptidyl peptidase-4 inhibitors versus intermediate-acting insulin or placebo for patients with type 2 diabetes failing two oral antihyperglycaemic agents: a systematic review and network meta-analysis
Abstract
Objective: To evaluate the effectiveness and safety of dipeptidyl peptidase-4 (DPP-4) inhibitors versus intermediate-acting insulin for adults with type 2 diabetes mellitus (T2DM) and poor glycaemic control despite treatment with two oral agents.
Setting: Studies were multicentre and multinational.
Participants: Ten studies including 2967 patients with T2DM.
Interventions: Studies that examined DPP-4 inhibitors compared with each other, intermediate-acting insulin, no treatment or placebo in patients with T2DM.
Primary and secondary outcome measures: Primary outcome was glycosylated haemoglobin (HbA1c). Secondary outcomes were healthcare utilisation, body weight, fractures, quality of life, microvascular complications, macrovascular complications, all-cause mortality, harms, cost and cost-effectiveness.
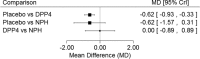
Results: 10 randomised clinical trials with 2967 patients were included after screening 5831 titles and abstracts, and 180 full-text articles. DPP-4 inhibitors significantly reduced HbA1c versus placebo in network meta-analysis (NMA; mean difference (MD) -0.62%, 95% CI -0.93% to -0.33%) and meta-analysis (MD -0.61%, 95% CI -0.81% to -0.41%), respectively. Significant differences in HbA1c were not observed for neutral protamine Hagedorn (NPH) insulin versus placebo and DPP-4 inhibitors versus NPH insulin in NMA. In meta-analysis, no significant differences were observed between DPP-4 inhibitors and placebo for severe hypoglycaemia, weight gain, cardiovascular disease, overall harms, treatment-related harms and mortality, although patients receiving DPP-4 inhibitors experienced less infections (relative risk 0.72, 95% CI 0.57 to 0.91).
Conclusions: DPP-4 inhibitors were superior to placebo in reducing HbA1c levels in adults with T2DM taking at least two oral agents. Compared with placebo, no safety signals were detected with DPP-4 inhibitors and there was a reduced risk of infection. There was no significant difference in HbA1c observed between NPH and placebo or NPH and DPP-4 inhibitors.
Trial registration number: PROSPERO # CRD42013003624.
Keywords: meta-analysis; systematic review.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
-
- Canadian Diabetes Association. Clinical practice guidelines for the prevention and management of diabetes in Canada. Toronto, ON: Canadian Diabetes Association, 2008.
-
- Nathan DM, Buse JB, Davidson MB et al. . Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203. 10.2337/dc08-9025 - DOI - PMC - PubMed
-
- Moher D, Shamseer L, Clarke M, et al. Reporting guidelines for systematic review protocols. 19th Cochrane Colloquium October 19–22. Madrid, Spain: Iberoamerican Cochrane Centre and Network, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
