Age-Associated Increase in BMP Signaling Inhibits Hippocampal Neurogenesis
- PMID: 25538007
- PMCID: PMC4818000
- DOI: 10.1002/stem.1943
Age-Associated Increase in BMP Signaling Inhibits Hippocampal Neurogenesis
Abstract
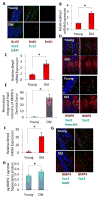
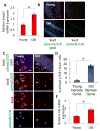
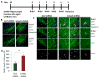
Hippocampal neurogenesis, the product of resident neural stem cell proliferation and differentiation, persists into adulthood but decreases with organismal aging, which may contribute to the age-related decline in cognitive function. The mechanisms that underlie this decrease in neurogenesis are not well understood, although evidence in general indicates that extrinsic changes in an aged stem cell niche can contribute to functional decline in old stem cells. Bone morphogenetic protein (BMP) family members are intercellular signaling proteins that regulate stem and progenitor cell quiescence, proliferation, and differentiation in various tissues and are likewise critical regulators of neurogenesis in young adults. Here, we establish that BMP signaling increases significantly in old murine hippocampi and inhibits neural progenitor cell proliferation. Furthermore, direct in vivo attenuation of BMP signaling via genetic and transgenic perturbations in aged mice led to elevated neural stem cell proliferation, and subsequent neurogenesis, in old hippocampi. Such advances in our understanding of mechanisms underlying decreased hippocampal neurogenesis with age may offer targets for the treatment of age-related cognitive decline.
Keywords: Adult stem cells; Aging; BMP signaling; Neural stem cell; Neurogenesis; Proliferation; Stem cell-microenvironment interactions.
© 2015 AlphaMed Press.
Conflict of interest statement
There are no conflict of interests.
Figures




References
-
- Ming G, Song H. Adult neurogenesis in the mammalian central nervous system. [Accessed October 17, 2013];ANNU REV NEUROSCI. 2005 28:223–50. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16022595. - PubMed
-
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? NAT REV NEUROSCI. 2010;11(5):339–350. Available at: http://dx.doi.org/10.1038/nrn2822. - DOI - PMC - PubMed
-
- Deng W, Saxe MD, Gallina IS, et al. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. [Accessed October 20, 2013];J NEUROSCI. 2009 29(43):13532–42. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2787190&tool=p.... - PMC - PubMed
-
- Oboti L, Savalli G, Giachino C, et al. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. EUR J NEUROSCI. 2009;29(4):679–692. Available at: http://dx.doi.org/10.1111/j.1460-9568.2009.06614.x. - DOI - PubMed
-
- Maslov AY, Barone Ta, Plunkett RJ, et al. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. [Accessed October 26, 2013];J NEUROSCI. 2004 24(7):1726–33. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14973255. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

