Highly diastereoselective and enantioselective olefin cyclopropanation using engineered myoglobin-based catalysts
- PMID: 25538035
- PMCID: PMC4470557
- DOI: 10.1002/anie.201409928
Highly diastereoselective and enantioselective olefin cyclopropanation using engineered myoglobin-based catalysts
Abstract
Using rational design, an engineered myoglobin-based catalyst capable of catalyzing the cyclopropanation of aryl-substituted olefins with catalytic proficiency (up to 46,800 turnovers) and excellent diastereo- and enantioselectivity (98-99.9%) was developed. This transformation could be carried out in the presence of up to 20 g L(-1) olefin substrate with no loss in diastereo- and/or enantioselectivity. Mutagenesis and mechanistic studies support a cyclopropanation mechanism mediated by an electrophilic, heme-bound carbene species and a model is provided to rationalize the stereopreference of the protein catalyst. This work shows that myoglobin constitutes a promising and robust scaffold for the development of biocatalysts with carbene-transfer reactivity.
Keywords: biocatalysis; carbenes; iron; protein engineering; small-ring systems.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
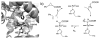
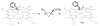
References
-
- Wilson ME, Whitesides GM. J Am Chem Soc. 1978;100:306–307.
- Collot J, Gradinaru J, Humbert N, Skander M, Zocchi A, Ward TR. J Am Chem Soc. 2003;125:9030–9031. - PubMed
- Reetz MT, Peyralans JJP, Maichele A, Fu Y, Maywald M. Chem Commun. 2006:4318–4320. - PubMed
- Abe S, Niemeyer J, Abe M, Takezawa Y, Ueno T, Hikage T, Erker G, Watanabe Y. J Am Chem Soc. 2008;130:10512–10514. - PubMed
- Mayer C, Gillingham DG, Ward TR, Hilvert D. Chem Commun. 2011;47:12068–12070. - PubMed
- Coelho PS, Brustad EM, Kannan A, Arnold FH. Science. 2013;339:307–310. - PubMed
- Singh R, Bordeaux M, Fasan R. ACS Catal. 2014;4:546–552. - PMC - PubMed
- Yang H, Srivastava P, Zhang C, Lewis JC. Chembiochem. 2014;15:223–227. - PMC - PubMed
-
- Wong HNC, Hon MY, Tse CW, Yip YC, Tanko J, Hudlicky T. Chem Rev. 1989;89:165–198.
-
- Doyle MP, Winchester WR, Hoorn JAA, Lynch V, Simonsen SH, Ghosh R. J Am Chem Soc. 1993;115:9968–9978.
- Nishiyama H, Itoh Y, Matsumoto H, Park SB, Itoh K. J Am Chem Soc. 1994;116:2223–2224.
- Uchida T, Irie R, Katsuki T. Synlett. 1999:1163–1165.
- Che CM, Huang JS, Lee FW, Li Y, Lai TS, Kwong HL, Teng PF, Lee WS, Lo WC, Peng SM, Zhou ZY. J Am Chem Soc. 2001;123:4119–4129. - PubMed
- Huang LY, Chen Y, Gao GY, Zhang XP. J Org Chem. 2003;68:8179–8184. - PubMed
-
- Callot HJ, Piechocki C. Tetrahedron Lett. 1980;21:3489–3492.
- Maxwell JL, Omalley S, Brown KC, Kodadek T. Organometallics. 1992;11:645–652.
- Wolf JR, Hamaker CG, Djukic JP, Kodadek T, Woo LK. J Am Chem Soc. 1995;117:9194–9199.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

