Differential effects on light chain amyloid formation depend on mutations and type of glycosaminoglycans
- PMID: 25538238
- PMCID: PMC4335233
- DOI: 10.1074/jbc.M114.615401
Differential effects on light chain amyloid formation depend on mutations and type of glycosaminoglycans
Abstract
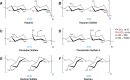
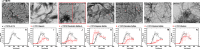
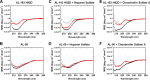
Amyloid light chain (AL) amyloidosis is a protein misfolding disease where immunoglobulin light chains sample partially folded states that lead to misfolding and amyloid formation, resulting in organ dysfunction and death. In vivo, amyloid deposits are found in the extracellular space and involve a variety of accessory molecules, such as glycosaminoglycans, one of the main components of the extracellular matrix. Glycosaminoglycans are a group of negatively charged heteropolysaccharides composed of repeating disaccharide units. In this study, we investigated the effect of glycosaminoglycans on the kinetics of amyloid fibril formation of three AL cardiac amyloidosis light chains. These proteins have similar thermodynamic stability but exhibit different kinetics of fibril formation. We also studied single restorative and reciprocal mutants and wild type germ line control protein. We found that the type of glycosaminoglycan has a different effect on the kinetics of fibril formation, and this effect seems to be associated with the natural propensity of each AL protein to form fibrils. Heparan sulfate accelerated AL-12, AL-09, κI Y87H, and AL-103 H92D fibril formation; delayed fibril formation for AL-103; and did not promote any fibril formation for AL-12 R65S, AL-103 delP95aIns, or κI O18/O8. Chondroitin sulfate A, on the other hand, showed a strong fibril formation inhibition for all proteins. We propose that heparan sulfate facilitates the formation of transient amyloidogenic conformations of AL light chains, thereby promoting amyloid formation, whereas chondroitin sulfate A kinetically traps partially unfolded intermediates, and further fibril elongation into fibrils is inhibited, resulting in formation/accumulation of oligomeric/protofibrillar aggregates.
Keywords: AL Amyloidosis; Amyloid; Amyloidosis; Fibril; Glycosaminoglycan; Immunoglobulin Fold; Immunoglobulin Light Chain; Light Chain Amyloidosis; Protein Misfolding.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures









References
-
- Merlini G., Stone M. J. (2006) Dangerous small B-cell clones. Blood 108, 2520–2530 - PubMed
-
- Kumar S. K., Dispenzieri A., Lacy M. Q., Hayman S. R., Buadi F. K., Zeldenrust S. R., Tan T., Sinha S., Leung N., Kyle R. A., Rajkumar S. V., Gertz M. A. (2011) Changes in serum-free light chain rather than intact monoclonal immunoglobulin levels predicts outcome following therapy in primary amyloidosis. Am. J. Hematol. 86, 251–255 - PMC - PubMed
-
- Abraham R. S., Geyer S. M., Price-Troska T. L., Allmer C., Kyle R. A., Gertz M. A., Fonseca R. (2003) Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood 101, 3801–3808 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

