Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study
- PMID: 25538262
- PMCID: PMC4334697
- DOI: 10.1158/1078-0432.CCR-14-2353
Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study
Abstract
Purpose: To examine the onset and outcome of ipilimumab-related hypophysitis and the response to treatment with systemic high-dose corticosteroids (HDS).
Experimental design: Twenty-five patients who developed ipilimumab-related hypophysitis were analyzed for the incidence, time to onset, time to resolution, frequency of resolution, and the effect of systemic HDS on clinical outcome. To calculate the incidence, the total number (187) of patients with metastatic melanoma treated with ipilimumab at Dana-Farber Cancer Institute (DFCI; Boston, MA) was retrieved from the DFCI oncology database. Comparisons between corticosteroid treatment groups were performed using the Fisher exact test. The distributions of overall survival were based on the method of Kaplan-Meier.
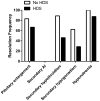
Results: The overall incidence of ipilimumab-related hypophysitis was 13%, with a higher rate in males (16.1%) than females (8.7%). The median time to onset of hypophysitis after initiation of ipilimumab treatment was 9 weeks (range, 5-36 weeks). Resolution of pituitary enlargement, secondary adrenal insufficiency, secondary hypothyroidism, male secondary hypogonadism, and hyponatremia occurred in 73%, 0%, 64%, 45%, and 92% of patients, respectively. Systemic HDS treatment did not improve the outcome of hypophysitis as measured by resolution frequency and time to resolution. One-year overall survival in the cohort of patients was 83%, and while it was slightly higher in patients who did not receive HDS, there was no statistically significant difference between treatment arms.
Conclusion: Systemic HDS therapy in patients with ipilimumab-related hypophysitis may not be indicated. Instead, supportive treatment of hypophysitis-related hormone deficiencies with the corresponding hormone replacement should be given.
©2014 American Association for Cancer Research.
Figures

References
-
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94. - PubMed
-
- Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

