The piriform cortex and human focal epilepsy
- PMID: 25538678
- PMCID: PMC4259123
- DOI: 10.3389/fneur.2014.00259
The piriform cortex and human focal epilepsy
Abstract
It is surprising that the piriform cortex, when compared to the hippocampus, has been given relatively little significance in human epilepsy. Like the hippocampus, it has a phylogenetically preserved three-layered cortex that is vulnerable to excitotoxic injury, has broad connections to both limbic and cortical areas, and is highly epileptogenic - being critical to the kindling process. The well-known phenomenon of early olfactory auras in temporal lobe epilepsy highlights its clinical relevance in human beings. Perhaps because it is anatomically indistinct and difficult to approach surgically, as it clasps the middle cerebral artery, it has, until now, been understandably neglected. In this review, we emphasize how its unique anatomical and functional properties, as primary olfactory cortex, predispose it to involvement in focal epilepsy. From recent convergent findings in human neuroimaging, clinical epileptology, and experimental animal models, we make the case that the piriform cortex is likely to play a facilitating and amplifying role in human focal epileptogenesis, and may influence progression to epileptic intractability.
Keywords: EEG-fMRI; area tempestas; claustrum; intracranial electrodes; olfaction; olfactory aura; pyriform; temporal lobe epilepsy.
Figures
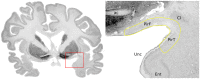
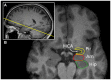
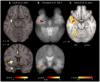
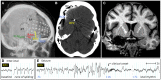
References
-
- Haberly LB. Neuronal circuitry in olfactory cortex: anatomy and functional implications. Chem Senses (1985) 10:219–3810.1093/chemse/10.2.219 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

