Membrane transporters and drought resistance - a complex issue
- PMID: 25538721
- PMCID: PMC4255493
- DOI: 10.3389/fpls.2014.00687
Membrane transporters and drought resistance - a complex issue
Abstract
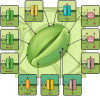
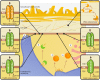
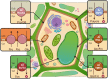
Land plants have evolved complex adaptation strategies to survive changes in water status in the environment. Understanding the molecular nature of such adaptive changes allows the development of rapid innovations to improve crop performance. Plant membrane transport systems play a significant role when adjusting to water scarcity. Here we put proteins participating in transmembrane allocations of various molecules in the context of stomatal, cuticular, and root responses, representing a part of the drought resistance strategy. Their role in the transport of signaling molecules, ions or osmolytes is summarized and the challenge of the forthcoming research, resulting from the recent discoveries, is highlighted.
Keywords: abscisic acid; drought avoidance; drought tolerance; transmembrane allocation; transport systems.
Figures

References
-
- Aroca R., Ruiz-Lozano J. (2012). “Regulation of root water uptake under drought stress conditions,” in Plant Responses to Drought Stress ed.Aroca R. (Berlin: Springer; ) 113–127 10.1007/978-3-642-32653-0_4 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

