Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids
- PMID: 25538905
- PMCID: PMC4260522
- DOI: 10.3389/fcimb.2014.00173
Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids
Abstract
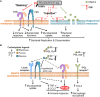
Mycobacterial pathogens, including Mycobacterium tuberculosis, the etiological agent of tuberculosis (TB), have evolved a remarkable ability to evade the immune system in order to survive and to colonize the host. Among the most important evasion strategies is the capacity of these bacilli to parasitize host macrophages, since these are major effector cells against intracellular pathogens that can be used as long-term cellular reservoirs. Mycobacterial pathogens employ an array of virulence factors that manipulate macrophage function to survive and establish infection. Until recently, however, the role of mycobacterial cell envelope lipids as virulence factors in macrophage subversion has remained elusive. Here, we will address exclusively the proposed role for phthiocerol dimycocerosates (DIM) in the modulation of the resident macrophage response and that of phenolic glycolipids (PGL) in the regulation of the recruitment and phenotype of incoming macrophage precursors to the site of infection. We will provide a unique perspective of potential additional functions for these lipids, and highlight obstacles and opportunities to further understand their role in the pathogenesis of TB and other mycobacterial diseases.
Keywords: immune responses; lipids; macrophages; mycobacteria; pathogens; virulence.
Figures

References
-
- Astarie-Dequeker C., Le Guyader L., Malaga W., Seaphanh F. K., Chalut C., Lopez A., et al. . (2009). Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 5:e1000289. 10.1371/journal.ppat.1000289 - DOI - PMC - PubMed
-
- Camacho L. R., Constant P., Raynaud C., Laneelle M. A., Triccas J. A., Gicquel B., et al. . (2001). Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 276, 19845–19854. 10.1074/jbc.M100662200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

