Commensal-dendritic-cell interaction specifies a unique protective skin immune signature
- PMID: 25539086
- PMCID: PMC4667810
- DOI: 10.1038/nature14052
Commensal-dendritic-cell interaction specifies a unique protective skin immune signature
Abstract
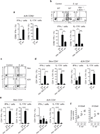
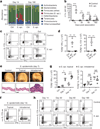
The skin represents the primary interface between the host and the environment. This organ is also home to trillions of microorganisms that play an important role in tissue homeostasis and local immunity. Skin microbial communities are highly diverse and can be remodelled over time or in response to environmental challenges. How, in the context of this complexity, individual commensal microorganisms may differentially modulate skin immunity and the consequences of these responses for tissue physiology remains unclear. Here we show that defined commensals dominantly affect skin immunity and identify the cellular mediators involved in this specification. In particular, colonization with Staphylococcus epidermidis induces IL-17A(+) CD8(+) T cells that home to the epidermis, enhance innate barrier immunity and limit pathogen invasion. Commensal-specific T-cell responses result from the coordinated action of skin-resident dendritic cell subsets and are not associated with inflammation, revealing that tissue-resident cells are poised to sense and respond to alterations in microbial communities. This interaction may represent an evolutionary means by which the skin immune system uses fluctuating commensal signals to calibrate barrier immunity and provide heterologous protection against invasive pathogens. These findings reveal that the skin immune landscape is a highly dynamic environment that can be rapidly and specifically remodelled by encounters with defined commensals, findings that have profound implications for our understanding of tissue-specific immunity and pathologies.
Figures







Comment in
-
Infection. Double skin protection.Nat Rev Immunol. 2015 Feb;15(2):68-9. doi: 10.1038/nri3811. Nat Rev Immunol. 2015. PMID: 25614313
-
S. epidermidis influence on host immunity: more than skin deep.Cell Host Microbe. 2015 Feb 11;17(2):143-4. doi: 10.1016/j.chom.2015.01.012. Cell Host Microbe. 2015. PMID: 25674978 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

