The proposed rule for U.S. clinical trial registration and results submission
- PMID: 25539444
- PMCID: PMC4344313
- DOI: 10.1056/NEJMsr1414226
The proposed rule for U.S. clinical trial registration and results submission
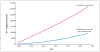
Figures

References
-
- Chalmers I. Underreporting research is scientific misconduct. JAMA. 1990;263:1405–8. - PubMed
-
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–60. - PubMed
-
- Vedula SS, Bero L, Scherer RW, Dickersin K. Outcome reporting in industry-sponsored trials of gabapentin for off-label use. N Engl J Med. 2009;361:1963–71. - PubMed
-
- Food and Drug Administration Amendments Act of 2007. Public Law 110–85. 2007 Sep 27; ( http://www.gpo.gov/fdsys/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
