BDNF release is required for the behavioral actions of ketamine
- PMID: 25539510
- PMCID: PMC4368871
- DOI: 10.1093/ijnp/pyu033
BDNF release is required for the behavioral actions of ketamine
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
Background: Recent studies demonstrate that the rapid antidepressant ketamine increases spine number and function in the medial prefrontal cortex (mPFC), and that these effects are dependent on activation of glutamate α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors and brain-derived neurotrophic factor (BDNF). In vitro studies also show that activation of AMPA receptors stimulates BNDF release via activation of L-type voltage-dependent calcium channels (VDCC).
Methods: Based on this evidence, we examined the role of BDNF release and the impact of L-type VDCCs on the behavioral actions of ketamine.
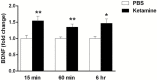
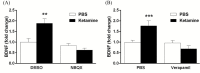
Results: The results demonstrate that infusion of a neutralizing BDNF antibody into the mPFC blocks the behavioral effects of ketamine in the forced swim test (FST). In addition, we show that pretreatment with nifedipine or verapamil, two structurally-different L-type calcium channel antagonists, blocks the behavioral effects of ketamine in the FST. Finally, we show that ketamine treatment stimulates BDNF release in primary cortical neurons and that this effect is blocked by inhibition of AMPA receptors or L-type VDCCs.
Conclusions: Taken together, these results indicate that the antidepressant effects of ketamine are mediated by activation of L-type VDCCs and the release of BDNF. They further elucidate the cellular mechanisms underlying this novel rapid-acting antidepressant.
Keywords: BDNF; L-type VDCC; depression; glutamate; ketamine.
© The Author 2014. Published by Oxford University Press on behalf of CINP.
Figures


References
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. - PubMed
-
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. (2001). Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50:260–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

