Ketamine's antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder
- PMID: 25539512
- PMCID: PMC4303351
- DOI: 10.1093/ijnp/pyu039
Ketamine's antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder
Erratum in
-
Erratum.Int J Neuropsychopharmacol. 2016 Apr 27;19(10):pyw031. doi: 10.1093/ijnp/pyw031. Int J Neuropsychopharmacol. 2016. PMID: 27207904 Free PMC article. No abstract available.
Abstract
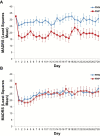
Background: A single subanesthetic infusion of the N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has rapid and potent antidepressant properties in treatment-resistant major depressive disorder (TRD). As a family history of an alcohol use disorder is a positive predictor of ketamine's antidepressant response and the strength of the association increases over time, we hypothesized that depressed subjects with a family history of an alcohol use disorder would have greater antidepressant durability and that riluzole would augment and/or extend ketamine's antidepressant efficacy.
Methods: Fifty-two TRD subjects received an open-label infusion of ketamine (0.5mg/kg over 40 minutes), and, four to six hours post-infusion, were randomized to either flexible-dose (100-200mg/day) riluzole or placebo in the following proportions: Family History Positive (FHP) riluzole (n = 10), FHP placebo (n = 9), Family History Negative (FHN) riluzole (n = 16), and FHN placebo (n = 17).
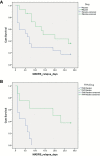
Results: FHP subjects randomized to placebo had a greater antidepressant response than FHN subjects; however, contrary to our initial hypothesis, there was no significant difference in antidepressant efficacy with riluzole. Although potentially underpowered, there was no difference in overall time-to-relapse based on randomization status (riluzole responders: n = 15, placebo responders: n = 17). Yet, time-to-relapse was longer in FHP placebo responders (n = 8) compared to FHN placebo responders (n = 9) with, again, no significant difference in time-to-relapse in FHP riluzole responders (n = 6) compared to FHN riluzole responders (n = 9).
Conclusions: Ketamine's extended antidepressant durability in FHP TRD should be considered in the design and analysis of ketamine depression trials.
Keywords: alcohol use disorder; family history; ketamine; major depressive disorder; riluzole.
Published by Oxford University Press on behalf of CINP 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
References
-
- Beck AT, Beamesderfer A. (1974). Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatr 7:151–169. - PubMed
-
- Beck AT, Kovacs M, Weissman A. (1979). Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol 47:343–352. - PubMed
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. - PubMed
-
- Blier P, Zigman D, Blier J. (2012). On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry 72:e11–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

