Impact of early enteral versus parenteral nutrition on mortality in patients requiring mechanical ventilation and catecholamines: study protocol for a randomized controlled trial (NUTRIREA-2)
- PMID: 25539571
- PMCID: PMC4307984
- DOI: 10.1186/1745-6215-15-507
Impact of early enteral versus parenteral nutrition on mortality in patients requiring mechanical ventilation and catecholamines: study protocol for a randomized controlled trial (NUTRIREA-2)
Abstract
Background: Nutritional support is crucial to the management of patients receiving invasive mechanical ventilation (IMV) and the most commonly prescribed treatment in intensive care units (ICUs). International guidelines consistently indicate that enteral nutrition (EN) should be preferred over parenteral nutrition (PN) whenever possible and started as early as possible. However, no adequately designed study has evaluated whether a specific nutritional modality is associated with decreased mortality. The primary goal of this trial is to assess the hypothesis that early first-line EN, as compared to early first-line PN, decreases day 28 all-cause mortality in patients receiving IMV and vasoactive drugs for shock.
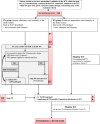
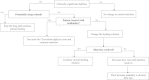
Methods/design: The NUTRIREA-2 study is a multicenter, open-label, parallel-group, randomized controlled trial comparing early PN versus early EN in critically ill patients requiring IMV for an expected duration of at least 48 hours, combined with vasoactive drugs, for shock. Patients will be allocated at random to first-line PN for at least 72 hours or to first-line EN. In both groups, nutritional support will be started within 24 hours after IMV initiation. Calorie targets will be 20 to 25 kcal/kg/day during the first week, then 25 to 30 kcal/kg/day thereafter. Patients receiving PN may be switched to EN after at least 72 hours in the event of shock resolution (no vasoactive drugs for 24 consecutive hours and arterial lactic acid level below 2 mmol/L). On day 7, all patients receiving PN and having no contraindications to EN will be switched to EN. In both groups, supplemental PN may be added to EN after day 7 in patients with persistent intolerance to EN and inadequate calorie intake. We plan to recruit 2,854 patients at 44 participating ICUs.
Discussion: The NUTRIREA-2 study is the first large randomized controlled trial designed to assess the hypothesis that early EN improves survival compared to early PN in ICU patients. Enrollment started on 22 March 2013 and is expected to end in November 2015.
Trial registration: ClinicalTrials.gov Identifier: NCT01802099 (registered 27 February 2013).
Figures

References
-
- Kreymann KG, Berger MM, Deutz NEP, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, DGEM (German Society for Nutritional Medicine) Ebner C, Hartl W, Heymann C, Spies C. ESPEN Guidelines on Enteral Nutrition: intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. - DOI - PubMed
-
- McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G, the A.S.P.E.N. Board of Directors, the American College of Critical Care Medicine Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. - DOI - PubMed
-
- Thuong M, Leteurtre S. [Experts recommendations of the Société de Réanimation de Langue Française: enteral nutrition in critical care] [Article in French] Reanimation. 2003;12:350–354. doi: 10.1016/S1624-0693(03)00079-3. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

