Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: a randomized, placebo-controlled, crossover trial
- PMID: 25539654
- PMCID: PMC4364572
- DOI: 10.1186/1471-2466-14-209
Aclidinium improves exercise endurance, dyspnea, lung hyperinflation, and physical activity in patients with COPD: a randomized, placebo-controlled, crossover trial
Abstract
Background: This study evaluated the effects of aclidinium bromide, a long-acting muscarinic antagonist indicated for maintenance treatment of chronic obstructive pulmonary disease (COPD), on exercise endurance, dyspnea, lung hyperinflation, and physical activity.
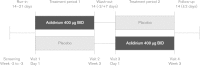
Methods: In this randomized, double-blind, crossover study, patients with stable COPD and moderate-to-severe airflow limitation received aclidinium 400 μg twice daily or placebo via Genuair®/Pressair(®a) for 3 weeks (2-week washout between treatment periods). The primary endpoint was change from baseline to Week 3 in endurance time, measured by constant work rate cycle ergometry testing at 75% peak incremental work rate. Changes from baseline in intensity of exertional dyspnea (Borg CR10 Scale®) and trough inspiratory capacity were secondary endpoints. Additional endpoints included changes from baseline in other spirometric, plethysmographic, and physical activity (assessed by objective accelerometer measurement) parameters. Efficacy endpoints were analyzed using an analysis of covariance model.
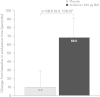
Results: In total, 112 patients were randomized and treated (mean age 60.3 years; mean post-bronchodilator forced expiratory volume in 1 s 1.7 L [56.7% predicted]; mean endurance time 485.7 s). After 3 weeks, endurance time was significantly increased with aclidinium versus placebo (treatment difference 58.5 s; p < 0.05). At Week 3, aclidinium significantly reduced dyspnea intensity at isotime during exercise (treatment difference -0.63; p < 0.05) and improved trough inspiratory capacity (treatment difference 78 mL; p < 0.05) versus placebo. Significant improvements in spirometric, plethysmographic, and some physical activity parameters were observed with aclidinium versus placebo.
Conclusions: These results suggest that aclidinium significantly improves exercise endurance, exertional dyspnea, hyperinflation, and physical activity in patients with COPD.
Trial registration: ClinicalTrials.gov identifier: NCT01471171; URL: http://www.clinicaltrials.gov.
Figures




References
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2466/14/209/prepub
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

