Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease
- PMID: 25539804
- PMCID: PMC4304998
- DOI: 10.1016/j.vaccine.2014.11.035
Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease
Abstract
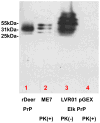
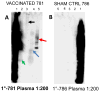
Prion disease is a unique category of illness, affecting both animals and humans, in which the underlying pathogenesis is related to a conformational change of a normal, self-protein called PrP(C) (C for cellular) to a pathological and infectious conformer known as PrP(Sc) (Sc for scrapie). Bovine spongiform encephalopathy (BSE), a prion disease believed to have arisen from feeding cattle with prion contaminated meat and bone meal products, crossed the species barrier to infect humans. Chronic wasting disease (CWD) infects large numbers of deer and elk, with the potential to infect humans. Currently no prionosis has an effective treatment. Previously, we have demonstrated we could prevent transmission of prions in a proportion of susceptible mice with a mucosal vaccine. In the current study, white-tailed deer were orally inoculated with attenuated Salmonella expressing PrP, while control deer were orally inoculated with vehicle attenuated Salmonella. Once a mucosal response was established, the vaccinated animals were boosted orally and locally by application of polymerized recombinant PrP onto the tonsils and rectal mucosa. The vaccinated and control animals were then challenged orally with CWD-infected brain homogenate. Three years post CWD oral challenge all control deer developed clinical CWD (median survival 602 days), while among the vaccinated there was a significant prolongation of the incubation period (median survival 909 days; p=0.012 by Weibull regression analysis) and one deer has remained CWD free both clinically and by RAMALT and tonsil biopsies. This negative vaccinate has the highest titers of IgA in saliva and systemic IgG against PrP. Western blots showed that immunoglobulins from this vaccinate react to PrP(CWD). We document the first partially successful vaccination for a prion disease in a species naturally at risk.
Keywords: Bovine spongiform encephalopathy; Chronic wasting disease; Immunization; Mucosal vaccination; Prion protein; Salmonella vaccine strain; White-tailed deer.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

