Hierarchy of non-glucose sugars in Escherichia coli
- PMID: 25539838
- PMCID: PMC4304618
- DOI: 10.1186/s12918-014-0133-z
Hierarchy of non-glucose sugars in Escherichia coli
Abstract
Background: Understanding how cells make decisions, and why they make the decisions they make, is of fundamental interest in systems biology. To address this, we study the decisions made by E. coli on which genes to express when presented with two different sugars. It is well-known that glucose, E. coli's preferred carbon source, represses the uptake of other sugars by means of global and gene-specific mechanisms. However, less is known about the utilization of glucose-free sugar mixtures which are found in the natural environment of E. coli and in biotechnology.
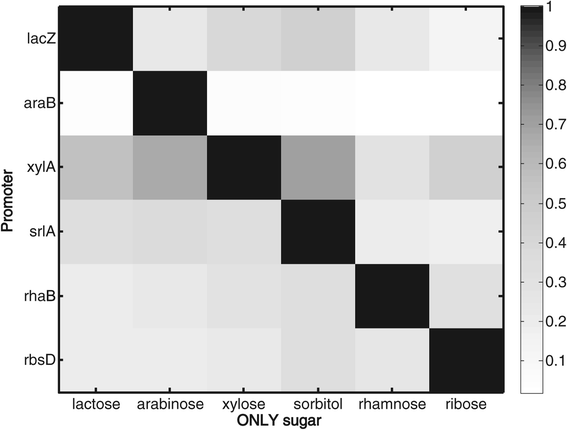
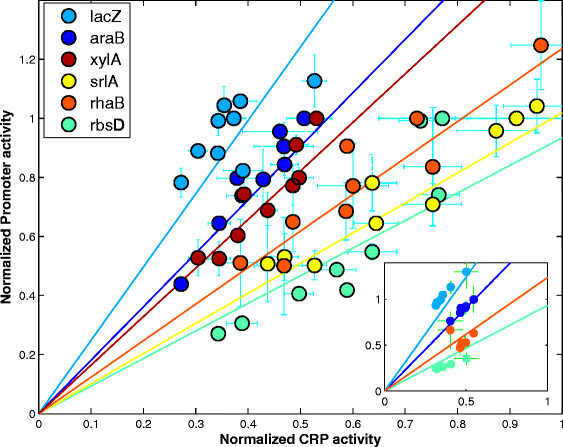
Results: Here, we combine experiment and theory to map the choices of E. coli among 6 different non-glucose carbon sources. We used robotic assays and fluorescence reporter strains to make precise measurements of promoter activity and growth rate in all pairs of these sugars. We find that the sugars can be ranked in a hierarchy: in a mixture of a higher and a lower sugar, the lower sugar system shows reduced promoter activity. The hierarchy corresponds to the growth rate supported by each sugar- the faster the growth rate, the higher the sugar on the hierarchy. The hierarchy is 'soft' in the sense that the lower sugar promoters are not completely repressed. Measurement of the activity of the master regulator CRP-cAMP shows that the hierarchy can be quantitatively explained based on differential activation of the promoters by CRP-cAMP. Comparing sugar system activation as a function of time in sugar pair mixtures at sub-saturating concentrations, we find cases of sequential activation, and also cases of simultaneous expression of both systems. Such simultaneous expression is not predicted by simple models of growth rate optimization, which predict only sequential activation. We extend these models by suggesting multi-objective optimization for both growing rapidly now and preparing the cell for future growth on the poorer sugar.
Conclusion: We find a defined hierarchy of sugar utilization, which can be quantitatively explained by differential activation by the master regulator cAMP-CRP. The present approach can be used to understand cell decisions when presented with mixtures of conditions.
Figures





References
-
- Monod J. Recherches Sur La Croissance Des Cultures Bacttriennes. Paris: Hermann and Cie; 1942.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

