Outcome of highly active antiretroviral therapy in HIV-infected Indian children
- PMID: 25539905
- PMCID: PMC4297378
- DOI: 10.1186/s12879-014-0701-2
Outcome of highly active antiretroviral therapy in HIV-infected Indian children
Abstract
Background: With the advent of highly active antiretroviral therapy (HAART), HIV infection has become a chronic condition in children with improved survival and quality of life. Reports on long term effectiveness of non-nucleoside reverse transcriptase inhibitor based HAART in HIV-infected children in developing countries are limited.
Methods: A chart review was conducted and children who received at least six months of HAART between 2004-2011 at All India Institute of Medical Sciences (AIIMS), Delhi were included. The clinical, immunological and virological responses to HAART were documented. Factors predicting non-adherence and non-response to treatment were described.
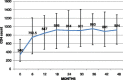
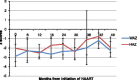
Results: One seventy five children (boys: 74.9%) were included in the study, with a median follow up of 43 (IQR: 17, 68) months. The median age at diagnosis was 119 (IQR: 75, 156) months. The median CD4 count at start of HAART was 340 cells/μL (IQR: 185,704), which increased to 924 cells/μL (IQR: 591,1278) at 48 months after HAART and plateaued at 749 (IQR: 542,1056) cells/ μL after 90 months of therapy. The weight for age (WAZ) and height for age (HAZ) z score both showed improvement with time after HAART initiation [baseline: WAZ -2.8 (IQR: -4,-1.6), HAZ -2.1 (IQR: -3.4,-0.69); at 42 months of therapy: WAZ -1.2 (IQR: -2.1, 0.01), HAZ -0.75(IQR: -1.6,-0.37)]. Adverse events were reported in 21 (12%) children. Non-adherence to therapy, treatment failure and death were noted in 35 (20%), 9 (5.1%) and 6 (3.4%) children respectively.
Conclusions: Our experience shows that HAART in HIV-infected children is effective, safe and is associated with good immunological and virological response as well as improvement in growth parameters.
Figures
Similar articles
-
Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study.BMC Pediatr. 2010 Aug 6;10:56. doi: 10.1186/1471-2431-10-56. BMC Pediatr. 2010. PMID: 20691045 Free PMC article.
-
Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth.Pediatrics. 2002 Feb;109(2):E25. doi: 10.1542/peds.109.2.e25. Pediatrics. 2002. PMID: 11826235
-
Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy.Pediatr Infect Dis J. 2009 Jun;28(6):488-92. doi: 10.1097/inf.0b013e318194eea6. Pediatr Infect Dis J. 2009. PMID: 19504731
-
Immunological, virological and clinical response to highly active antiretroviral therapy treatment regimens in a complete clinic population. Royal Free Centre for HIV Medicine.AIDS. 2000 Jul 28;14(11):1545-52. doi: 10.1097/00002030-200007280-00010. AIDS. 2000. PMID: 10983641 Clinical Trial.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
Cited by
-
Predictors of first-line antiretroviral treatment failure among children on antiretroviral therapy at the University of Gondar comprehensive specialised hospital, North-west, Ethiopia: a 14-year long-term follow-up study.BMJ Open. 2022 Dec 20;12(12):e064354. doi: 10.1136/bmjopen-2022-064354. BMJ Open. 2022. PMID: 36600440 Free PMC article.
-
Heterogeneity and Hierarchy of Immune Response to Primary Immunization in HIV-Infected Children on HAART and the Impact of an Additional Dose of Vaccine.Indian J Pediatr. 2025 Feb;92(2):181-184. doi: 10.1007/s12098-024-05148-4. Epub 2024 May 27. Indian J Pediatr. 2025. PMID: 38801497
-
Counseling for improving adherence to antiretroviral treatment: a systematic review.AIDS Care. 2019 Jan;31(1):4-13. doi: 10.1080/09540121.2018.1533224. Epub 2018 Oct 11. AIDS Care. 2019. PMID: 30309239 Free PMC article.
-
Incidence and Predictors of Treatment Failure Among Children Receiving First-Line Antiretroviral Treatment in General Hospitals of Two Zones, Tigray, Ethiopia, 2019.Pediatric Health Med Ther. 2020 Mar 6;11:85-94. doi: 10.2147/PHMT.S243656. eCollection 2020. Pediatric Health Med Ther. 2020. PMID: 32189973 Free PMC article.
-
Incidence and risk factors of first-line antiretroviral treatment failure among human immunodeficiency virus-infected children in Amhara regional state, Ethiopia: a retrospective follow-up study.BMJ Open. 2018 Apr 5;8(4):e019181. doi: 10.1136/bmjopen-2017-019181. BMJ Open. 2018. PMID: 29626042 Free PMC article.
References
-
- Foster CA, Lyall EGH. Children with HIV: improved mortality and morbidity with combination antiretroviral therapy. Curr Opin Infect Dis. 2005;18:253–259. doi: 10.1097/01.qco.0000168387.24142.cf. - DOI - PubMed
-
- Lodha R, Upadhyay A, Kabra SK. Antiretroviraltherapy in HIV-1infectedchildren. Indian Pediatr. 2005;42:789–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials