Sequence-dependent off-target inhibition of TLR7/8 sensing by synthetic microRNA inhibitors
- PMID: 25539920
- PMCID: PMC4333393
- DOI: 10.1093/nar/gku1343
Sequence-dependent off-target inhibition of TLR7/8 sensing by synthetic microRNA inhibitors
Abstract
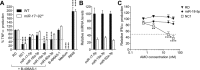
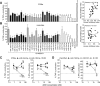
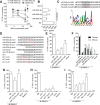
Anti-microRNA (miRNA) oligonucleotides (AMOs) with 2'-O-Methyl (2'OMe) residues are commonly used to study miRNA function and can achieve high potency, with low cytotoxicity. Not withstanding this, we demonstrate the sequence-dependent capacity of 2'OMe AMOs to inhibit Toll-like receptor (TLR) 7 and 8 sensing of immunostimulatory RNA, independent of their miRNA-targeting function. Through a screen of 29 AMOs targeting common miRNAs, we found a subset of sequences highly inhibitory to TLR7 sensing in mouse macrophages. Interspecies conservation of this inhibitory activity was confirmed on TLR7/8 activity in human peripheral blood mononuclear cells. Significantly, we identified a core motif governing the inhibitory activity of these AMOs, which is present in more than 50 AMOs targeted to human miRNAs in miRBaseV20. DNA/locked nucleic acids (LNA) AMOs synthesized with a phosphorothioate backbone also inhibited TLR7 sensing in a sequence-dependent manner, demonstrating that the off-target effects of AMOs are not restricted to 2'OMe modification. Taken together, our work establishes the potential for off-target effects of AMOs on TLR7/8 function, which should be taken into account in their therapeutic development and in vivo application.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Assessing the Off-Target Effects of miRNA Inhibitors on Innate Immune Toll-Like Receptors.Methods Mol Biol. 2017;1517:127-135. doi: 10.1007/978-1-4939-6563-2_9. Methods Mol Biol. 2017. PMID: 27924479
-
Rational design of antisense oligonucleotides modulating the activity of TLR7/8 agonists.Nucleic Acids Res. 2020 Jul 27;48(13):7052-7065. doi: 10.1093/nar/gkaa523. Nucleic Acids Res. 2020. PMID: 32544249 Free PMC article.
-
2'-O-methyl-modified RNAs act as TLR7 antagonists.Mol Ther. 2007 Sep;15(9):1663-9. doi: 10.1038/sj.mt.6300240. Epub 2007 Jun 19. Mol Ther. 2007. PMID: 17579574
-
Anti-miRNA oligonucleotides: A comprehensive guide for design.RNA Biol. 2018 Mar 4;15(3):338-352. doi: 10.1080/15476286.2018.1445959. Epub 2018 Mar 23. RNA Biol. 2018. PMID: 29570036 Free PMC article. Review.
-
Inhibition of microRNA with antisense oligonucleotides.Methods. 2008 Jan;44(1):55-60. doi: 10.1016/j.ymeth.2007.11.001. Methods. 2008. PMID: 18158133 Review.
Cited by
-
Essential role of miR-200c in regulating self-renewal of breast cancer stem cells and their counterparts of mammary epithelium.BMC Cancer. 2015 Sep 23;15:645. doi: 10.1186/s12885-015-1655-5. BMC Cancer. 2015. PMID: 26400441 Free PMC article.
-
Tissue nanotransfection causes tumor regression by its effect on nanovesicle cargo that alters microenvironmental macrophage state.Mol Ther. 2023 May 3;31(5):1402-1417. doi: 10.1016/j.ymthe.2022.11.003. Epub 2022 Nov 14. Mol Ther. 2023. PMID: 36380587 Free PMC article.
-
Oligonucleotide-Based Modulation of Macrophage Polarization: Emerging Strategies in Immunotherapy.Immun Inflamm Dis. 2025 May;13(5):e70200. doi: 10.1002/iid3.70200. Immun Inflamm Dis. 2025. PMID: 40325939 Free PMC article. Review.
-
Cathepsin B-activatable cyclic antisense oligonucleotides for cell-specific target gene knockdown in vitro and in vivo.Mol Ther Nucleic Acids. 2023 Jul 25;33:548-558. doi: 10.1016/j.omtn.2023.07.022. eCollection 2023 Sep 12. Mol Ther Nucleic Acids. 2023. PMID: 37588686 Free PMC article.
-
Pigmy MicroRNA: surveillance cops in Therapies kingdom.Mol Med. 2016 Dec;22:759-775. doi: 10.2119/molmed.2016.00136. Epub 2016 Sep 28. Mol Med. 2016. PMID: 27704139 Free PMC article.
References
-
- Selbach M., Schwanhausser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. - PubMed
-
- Eulalio A., Huntzinger E., Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. - PubMed
-
- Lennox K.A., Behlke M.A. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18:1111–1120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

