The organic anion transporter (OAT) family: a systems biology perspective
- PMID: 25540139
- PMCID: PMC4281586
- DOI: 10.1152/physrev.00025.2013
The organic anion transporter (OAT) family: a systems biology perspective
Abstract
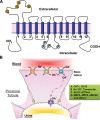
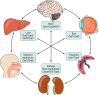
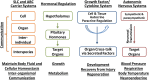
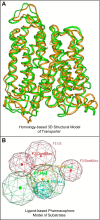
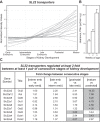
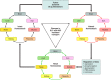
The organic anion transporter (OAT) subfamily, which constitutes roughly half of the SLC22 (solute carrier 22) transporter family, has received a great deal of attention because of its role in handling of common drugs (antibiotics, antivirals, diuretics, nonsteroidal anti-inflammatory drugs), toxins (mercury, aristolochic acid), and nutrients (vitamins, flavonoids). Oats are expressed in many tissues, including kidney, liver, choroid plexus, olfactory mucosa, brain, retina, and placenta. Recent metabolomics and microarray data from Oat1 [Slc22a6, originally identified as NKT (novel kidney transporter)] and Oat3 (Slc22a8) knockouts, as well as systems biology studies, indicate that this pathway plays a central role in the metabolism and handling of gut microbiome metabolites as well as putative uremic toxins of kidney disease. Nuclear receptors and other transcription factors, such as Hnf4α and Hnf1α, appear to regulate the expression of certain Oats in conjunction with phase I and phase II drug metabolizing enzymes. Some Oats have a strong selectivity for particular signaling molecules, including cyclic nucleotides, conjugated sex steroids, odorants, uric acid, and prostaglandins and/or their metabolites. According to the "Remote Sensing and Signaling Hypothesis," which is elaborated in detail here, Oats may function in remote interorgan communication by regulating levels of signaling molecules and key metabolites in tissues and body fluids. Oats may also play a major role in interorganismal communication (via movement of small molecules across the intestine, placental barrier, into breast milk, and volatile odorants into the urine). The role of various Oat isoforms in systems physiology appears quite complex, and their ramifications are discussed in the context of remote sensing and signaling.
Copyright © 2015 the American Physiological Society.
Figures







References
-
- Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 22: 86–89, 2012. - PubMed
-
- Ahn SY, Bhatnagar V. Update on the molecular physiology of organic anion transporters. Curr Opin Nephrol Hypertens 17: 499–505, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

