The gastrointestinal microbiota and colorectal cancer
- PMID: 25540232
- PMCID: PMC4346754
- DOI: 10.1152/ajpgi.00360.2012
The gastrointestinal microbiota and colorectal cancer
Abstract
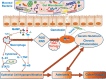
The human gut is home to a complex and diverse microbiota that contributes to the overall homeostasis of the host. Increasingly, the intestinal microbiota is recognized as an important player in human illness such as colorectal cancer (CRC), inflammatory bowel diseases, and obesity. CRC in itself is one of the major causes of cancer mortality in the Western world. The mechanisms by which bacteria contribute to CRC are complex and not fully understood, but increasing evidence suggests a link between the intestinal microbiota and CRC as well as diet and inflammation, which are believed to play a role in carcinogenesis. It is thought that the gut microbiota interact with dietary factors to promote chronic inflammation and CRC through direct influence on host cell physiology, cellular homeostasis, energy regulation, and/or metabolism of xenobiotics. This review provides an overview on the role of commensal gut microbiota in the development of human CRC and explores its association with diet and inflammation.
Keywords: bacterial metabolites; colorectal adenoma; diet; inflammation.
Copyright © 2015 the American Physiological Society.
Figures

References
-
- Araki Y, Andoh A, Takizawa J, Takizawa W, Fujiyama Y. Clostridium butyricum, a probiotic derivative, suppresses dextran sulfate sodium-induced experimental colitis in rats. Int J Mol Med 13: 577–580, 2004. - PubMed
-
- Arends MJ. Pathways of colorectal carcinogenesis. Appl Immunohistochem Mol Mmorphol 21: 97–102, 2013. - PubMed
-
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120–123, 2012. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

