Two activities of long-chain acyl-coenzyme A synthetase are involved in lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis
- PMID: 25540329
- PMCID: PMC4326746
- DOI: 10.1104/pp.114.250365
Two activities of long-chain acyl-coenzyme A synthetase are involved in lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis
Abstract
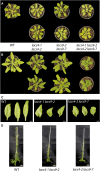
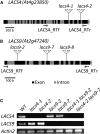
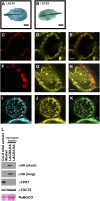
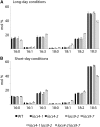
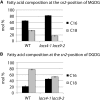
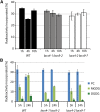
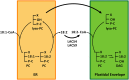
In plants, fatty acids are synthesized within the plastid and need to be distributed to the different sites of lipid biosynthesis within the cell. Free fatty acids released from the plastid need to be converted to their corresponding coenzyme A thioesters to become metabolically available. This activation is mediated by long-chain acyl-coenzyme A synthetases (LACSs), which are encoded by a family of nine genes in Arabidopsis (Arabidopsis thaliana). So far, it has remained unclear which of the individual LACS activities are involved in making plastid-derived fatty acids available to cytoplasmic glycerolipid biosynthesis. Because of its unique localization at the outer envelope of plastids, LACS9 was regarded as a candidate for linking plastidial fatty export and cytoplasmic use. However, data presented in this study show that LACS9 is involved in fatty acid import into the plastid. The analyses of mutant lines revealed strongly overlapping functions of LACS4 and LACS9 in lipid trafficking from the endoplasmic reticulum to the plastid. In vivo labeling experiments with lacs4 lacs9 double mutants suggest strongly reduced synthesis of endoplasmic reticulum-derived lipid precursors, which are required for the biosynthesis of glycolipids in the plastids. In conjunction with this defect, double-mutant plants accumulate significant amounts of linoleic acid in leaf tissue.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Andersson MX, Goksör M, Sandelius AS (2007) Optical manipulation reveals strong attracting forces at membrane contact sites between endoplasmic reticulum and chloroplasts. J Biol Chem 282: 1170–1174 - PubMed
-
- Aronsson H, Jarvis P (2002) A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett 529: 215–220 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

