Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein
- PMID: 25540360
- PMCID: PMC4325748
- DOI: 10.1128/JVI.03117-14
Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein
Abstract
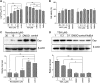
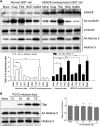
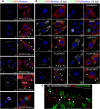
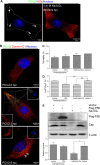
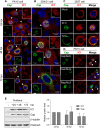
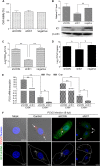
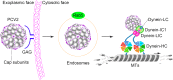
Microtubule transport of circovirus from the periphery of the cell to the nucleus is essential for viral replication in early infection. How the microtubule is recruited to the viral cargo remains unclear. In this study, we observed that circovirus trafficking is dependent on microtubule polymerization and that incoming circovirus particles colocalize with cytoplasmic dynein and endosomes. However, circovirus binding to dynein was independent of the presence of microtubular α-tubulin and translocation of cytoplasmic dynein into the nucleus. The circovirus capsid (Cap) subunit enhanced microtubular acetylation and directly interacted with intermediate chain 1 (IC1) of dynein. N-terminal residues 42 to 100 of the Cap viral protein were required for efficient binding to the dynein IC1 subunit and for retrograde transport. Knockdown of IC1 decreased virus transport and replication. These results demonstrate that Cap is a direct ligand of the cytoplasmic dynein IC1 subunit and an inducer of microtubule α-tubulin acetylation. Furthermore, Cap recruits the host dynein/microtubule machinery to facilitate transport toward the nucleus by an endosomal mechanism distinct from that used for physiological dynein cargo.
Importance: Incoming viral particles hijack the intracellular trafficking machinery of the host in order to migrate from the cell surface to the replication sites. Better knowledge of the interaction between viruses and virus proteins and the intracellular trafficking machinery may provide new targets for antiviral therapies. Currently, little is known about the molecular mechanisms of circovirus transport. Here, we report that circovirus particles enter early endosomes and utilize the microtubule-associated molecular motor dynein to travel along microtubules. The circovirus capsid subunit enhances microtubular acetylation, and N-terminal residues 42 to 100 directly interact with the dynein IC1 subunit during retrograde transport. These findings highlight a mechanism whereby circoviruses recruit dynein for transport to the nucleus via the dynein/microtubule machinery.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Biagini P, Bendinelli M, Hino S, Kakkola L, Mankertz A, Niel C, Okamoto H, Raidal S, Teo CG, Todd D. 2012. Circoviridae, p 343–349 InKing AMQ, Lefkowitz E, Adams MJ, Carstens EB (ed), Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

