A multivalent clade C HIV-1 Env trimer cocktail elicits a higher magnitude of neutralizing antibodies than any individual component
- PMID: 25540368
- PMCID: PMC4325749
- DOI: 10.1128/JVI.03331-14
A multivalent clade C HIV-1 Env trimer cocktail elicits a higher magnitude of neutralizing antibodies than any individual component
Abstract
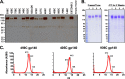
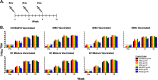
The sequence diversity of human immunodeficiency virus type 1 (HIV-1) presents a formidable challenge to the generation of an HIV-1 vaccine. One strategy to address such sequence diversity and to improve the magnitude of neutralizing antibodies (NAbs) is to utilize multivalent mixtures of HIV-1 envelope (Env) immunogens. Here we report the generation and characterization of three novel, acute clade C HIV-1 Env gp140 trimers (459C, 405C, and 939C), each with unique antigenic properties. Among the single trimers tested, 459C elicited the most potent NAb responses in vaccinated guinea pigs. We evaluated the immunogenicity of various mixtures of clade C Env trimers and found that a quadrivalent cocktail of clade C trimers elicited a greater magnitude of NAbs against a panel of tier 1A and 1B viruses than any single clade C trimer alone, demonstrating that the mixture had an advantage over all individual components of the cocktail. These data suggest that vaccination with a mixture of clade C Env trimers represents a promising strategy to augment vaccine-elicited NAb responses.
Importance: It is currently not known how to generate potent NAbs to the diverse circulating HIV-1 Envs by vaccination. One strategy to address this diversity is to utilize mixtures of different soluble HIV-1 envelope proteins. In this study, we generated and characterized three distinct, novel, acute clade C soluble trimers. We vaccinated guinea pigs with single trimers as well as mixtures of trimers, and we found that a mixture of four trimers elicited a greater magnitude of NAbs than any single trimer within the mixture. The results of this study suggest that further development of Env trimer cocktails is warranted.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. - DOI - PMC - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

