Alpha-defensin HD5 inhibits furin cleavage of human papillomavirus 16 L2 to block infection
- PMID: 25540379
- PMCID: PMC4325740
- DOI: 10.1128/JVI.02901-14
Alpha-defensin HD5 inhibits furin cleavage of human papillomavirus 16 L2 to block infection
Abstract
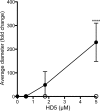
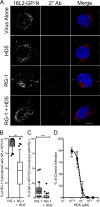
Human papillomavirus (HPV) is a significant oncogenic virus, but the innate immune response to HPV is poorly understood. Human α-defensin 5 (HD5) is an innate immune effector peptide secreted by epithelial cells in the genitourinary tract. HD5 is broadly antimicrobial, exhibiting potent antiviral activity against HPV at physiologic concentrations; however, the specific mechanism of HD5-mediated inhibition against HPV is unknown. During infection, the HPV capsid undergoes several critical cell-mediated viral protein processing steps, including unfolding and cleavage of the minor capsid protein L2 by host cyclophilin B and furin. Using HPV16 pseudovirus, we show that HD5 interacts directly with the virus and inhibits the furin-mediated cleavage of L2 at the cell surface during infection at a step downstream of the cyclophilin B-mediated unfolding of L2. Importantly, HD5 does not affect the enzymatic activity of furin directly. Thus, our data support a model in which HD5 prevents furin from accessing L2 by occluding the furin cleavage site via direct binding to the viral capsid.
Importance: Our study elucidates a new antiviral action for α-defensins against nonenveloped viruses in which HD5 directly interferes with a critical host-mediated viral processing step, furin cleavage of L2, at the cell surface. Blocking this key event has deleterious effects on the intracellular steps of virus infection. Thus, in addition to informing the antiviral mechanisms of α-defensins, our studies highlight the critical role of furin cleavage in HPV entry. Innate immune control, mediated in part by α-defensins expressed in the genital mucosa, may influence susceptibility to HPV infections that lead to cervical cancer. Moreover, understanding the mechanism of these natural antivirals may inform the design of therapeutics to limit HPV infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

