Chicken and duck myotubes are highly susceptible and permissive to influenza virus infection
- PMID: 25540384
- PMCID: PMC4325732
- DOI: 10.1128/JVI.03421-14
Chicken and duck myotubes are highly susceptible and permissive to influenza virus infection
Abstract
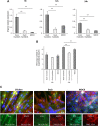
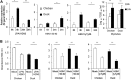
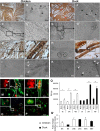
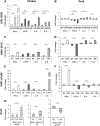
Skeletal muscle, at 30 to 40% of body mass, is the most abundant soft tissue in the body. Besides its primary function in movement and posture, skeletal muscle is a significant innate immune organ with the capacity to produce cytokines and chemokines and respond to proinflammatory cytokines. Little is known about the role of skeletal muscle during systemic influenza A virus infection in any host and particularly avian species. Here we used primary chicken and duck multinucleated myotubes to examine their susceptibility and innate immune response to influenza virus infections. Both chicken and duck myotubes expressed avian and human sialic acid receptors and were readily susceptible to low-pathogenicity (H2N3 A/mallard duck/England/7277/06) and high-pathogenicity (H5N1 A/turkey/England/50-92/91 and H5N1 A/turkey/Turkey/1/05) avian and human H1N1 (A/USSR/77) influenza viruses. Both avian host species produced comparable levels of progeny H5N1 A/turkey/Turkey/1/05 virus. Notably, the rapid accumulation of viral nucleoprotein and matrix (M) gene RNA in chicken and duck myotubes was accompanied by extensive cytopathic damage with marked myotube apoptosis (widespread microscopic blebs, caspase 3/7 activation, and annexin V binding at the plasma membrane). Infected chicken myotubes produced significantly higher levels of proinflammatory cytokines than did the corresponding duck cells. Additionally, in chicken myotubes infected with H5N1 viruses, the induction of interferon beta (IFN-β) and IFN-inducible genes, including the melanoma differentiation-associated protein 5 (MDA-5) gene, was relatively weak compared to infection with the corresponding H2N3 virus. Our findings highlight that avian skeletal muscle fibers are capable of productive influenza virus replication and are a potential tissue source of infection.
Importance: Infection with high-pathogenicity H5N1 viruses in ducks is often asymptomatic, and skeletal muscle from such birds could be a source of infection of humans and animals. Little is known about the ability of influenza A viruses to replicate in avian skeletal muscle fibers. We show here that cultured chicken and duck myotubes were highly susceptible to infection with both low- and high-pathogenicity avian influenza viruses. Infected myotubes of both avian species displayed rapid virus accumulation, apoptosis, and extensive cellular damage. Our results indicate that avian skeletal muscle fibers of chicken and duck could be significant contributors to progeny production of highly pathogenic H5N1 viruses.
Copyright © 2015, Baquero-Perez et al.
Figures

References
-
- Guan Y, Smith GJD, Webby R, Webster RG. 2009. Molecular epidemiology of H5N1 avian influenza. Rev Sci Tech 28:39–47. - PubMed
-
- Sims LS, Brown IH. 2008. Multi-continental epizootics of H5N1 highly pathogenic avian influenza 1996–2007, p 251–286 InSwayne DE. (ed), Avian influenza. Blackwell Publishing, Oxford, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

