Endogenous human milk peptide release is greater after preterm birth than term birth
- PMID: 25540406
- PMCID: PMC4336528
- DOI: 10.3945/jn.114.203646
Endogenous human milk peptide release is greater after preterm birth than term birth
Abstract
Background: Hundreds of naturally occurring milk peptides are present in term human milk. Preterm milk is produced before complete maturation of the mammary gland, which could change milk synthesis and secretion processes within the mammary gland, leading to differences in protein expression and enzymatic activity, thereby resulting in an altered peptide profile.
Objective: This study examined differences in peptides present between milk from women delivering at term and women delivering prematurely.
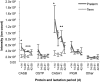
Methods: Nano-LC tandem mass spectrometry was employed to identify naturally occurring peptides and compare their abundances between term and preterm human milk samples at multiple time points over lactation. Term milk samples were collected from 8 mothers and preterm milk was collected from 14 mothers. The 28 preterm and 32 term human milk samples were divided into 4 groups based on day of collection (<14, 14-28, 29-41, and 42-58 d).
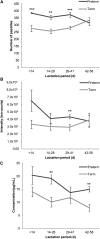
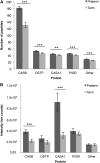
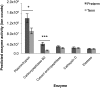
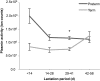
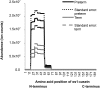
Results: Preterm milk peptide counts, ion abundance, and concentration were significantly higher in preterm milk than term milk. Bioinformatic analysis of the cleavage sites for peptides identified suggested that plasmin was more active in preterm milk than term milk and that cytosol aminopeptidase and carboxypeptidase B2 likely contribute to extensive milk protein breakdown. Many identified milk peptides in both term and preterm milk overlapped with known functional peptides, including antihypertensive, antimicrobial, and immunomodulatory peptides.
Conclusion: The high protein degradation by endogenous proteases in preterm milk might attenuate problems because of the preterm infant's immature digestive system. This trial was registered at clinicaltrials.gov as NCT01817127.
Keywords: carboxypeptidase B2; cytosol aminopeptidase; functional peptide; human milk; mass spectrometry; peptidomics; plasmin; premature; term.
© 2015 American Society for Nutrition.
Conflict of interest statement
Author disclosures: DC Dallas, CJ Smink, RC Robinson, T Tian, A Guerrero, EA Parker, JT Smilowitz, KA Hettinga, MA Underwood, CB Lebrilla, JB German, and D Barile, no conflicts of interest.
Figures

References
-
- Ferranti P, Traisci MV, Picariello G, Nasi A, Boschi V, Siervo M, Falconi C, Chianese L, Addeo F. Casein proteolysis in human milk: tracing the pattern of casein breakdown and the formation of potential bioactive peptides. J Dairy Res 2004;71:74–87. - PubMed
-
- Heegaard CW, Larsen LB, Rasmussen LK, Højberg KE, Petersen TE, Andreasen PA. Plasminogen activation system in human milk. J Pediatr Gastroenterol Nutr 1997;25:159–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

