Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells
- PMID: 25540431
- PMCID: PMC4325843
- DOI: 10.1091/mbc.E14-08-1307
Clathrin-dependent entry and vesicle-mediated exocytosis define insulin transcytosis across microvascular endothelial cells
Abstract
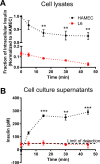
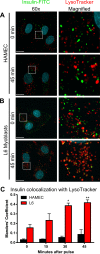
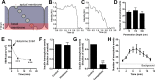
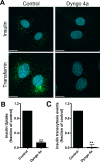
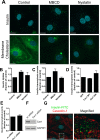
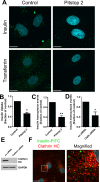
Transport of insulin across the microvasculature is necessary to reach its target organs (e.g., adipose and muscle tissues) and is rate limiting in insulin action. Morphological evidence suggests that insulin enters endothelial cells of the microvasculature, and studies with large vessel-derived endothelial cells show insulin uptake; however, little is known about the actual transcytosis of insulin and how this occurs in the relevant microvascular endothelial cells. We report an approach to study insulin transcytosis across individual, primary human adipose microvascular endothelial cells (HAMECs), involving insulin uptake followed by vesicle-mediated exocytosis visualized by total internal reflection fluorescence microscopy. In this setting, fluorophore-conjugated insulin exocytosis depended on its initial binding and uptake, which was saturable and much greater than in muscle cells. Unlike its degradation within muscle cells, insulin was stable within HAMECs and escaped lysosomal colocalization. Insulin transcytosis required dynamin but was unaffected by caveolin-1 knockdown or cholesterol depletion. Instead, insulin transcytosis was significantly inhibited by the clathrin-mediated endocytosis inhibitor Pitstop 2 or siRNA-mediated clathrin depletion. Accordingly, insulin internalized for 1 min in HAMECs colocalized with clathrin far more than with caveolin-1. This study constitutes the first evidence of vesicle-mediated insulin transcytosis and highlights that its initial uptake is clathrin dependent and caveolae independent.
© 2015 Azizi et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007a;100:158–173. - PubMed
-
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007b;100:174–190. - PubMed
-
- Armstrong SM, Khajoee V, Wang C, Wang T, Tigdi J, Yin J, Kuebler WM, Gillrie M, Davis SP, Ho M, et al. Co-regulation of transcellular and paracellular leak across microvascular endothelium by dynamin and Rac. Am J Pathol. 2012;180:1308–1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

