Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology
- PMID: 25540432
- PMCID: PMC4325842
- DOI: 10.1091/mbc.E14-08-1303
Seipin performs dissectible functions in promoting lipid droplet biogenesis and regulating droplet morphology
Abstract
Seipin is necessary for both adipogenesis and lipid droplet (LD) organization in nonadipose tissues; however, its molecular function is incompletely understood. Phenotypes in the seipin-null mutant of Saccharomyces cerevisiae include aberrant droplet morphology (endoplasmic reticulum-droplet clusters and size heterogeneity) and sensitivity of droplet size to changes in phospholipid synthesis. It has not been clear, however, whether seipin acts in initiation of droplet synthesis or at a later step. Here we utilize a system of de novo droplet formation to show that the absence of seipin results in a delay in droplet appearance with concomitant accumulation of neutral lipid in membranes. We also demonstrate that seipin is required for vectorial budding of droplets toward the cytoplasm. Furthermore, we find that the normal rate of droplet initiation depends on 14 amino acids at the amino terminus of seipin, deletion of which results in fewer, larger droplets that are consistent with a delay in initiation but are otherwise normal in morphology. Importantly, other functions of seipin, namely vectorial budding and resistance to inositol, are retained in this mutant. We conclude that seipin has dissectible roles in both promoting early LD initiation and in regulating LD morphology, supporting its importance in LD biogenesis.
© 2015 Cartwright et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

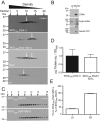

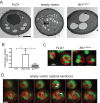


 , p < 0.0001 by one-way ANOVA with correction for multiple comparisons. (E) Time-lapse fluorescence microscopy of cells embedded into agar after 1 h of galactose induction in liquid culture at 27–29°C. Images taken in 10-min increments (see Supplemental Videos S1–S3); representative projections of 30-min increment montages are shown. Note that cells were precultured at high density to prevent division during imaging. (F) Percent of cells that displayed at least one FB over the course of the time lapse. Error bars represent SEMs from three independent experiments. ***, p < 0.001 by one-way ANOVA with correction for multiple comparisons. (G) Histogram of time to first appearance for each droplet. (H) Average intensity curves for FBs during the time lapse. Time 0 defined as the frame before first appearance of the droplet. Error bars represent SEMs from average droplet intensity values per time point over three independent experiments. Absolute intensity values are likely underestimated due to bleaching. Scale bars: 5 µm
, p < 0.0001 by one-way ANOVA with correction for multiple comparisons. (E) Time-lapse fluorescence microscopy of cells embedded into agar after 1 h of galactose induction in liquid culture at 27–29°C. Images taken in 10-min increments (see Supplemental Videos S1–S3); representative projections of 30-min increment montages are shown. Note that cells were precultured at high density to prevent division during imaging. (F) Percent of cells that displayed at least one FB over the course of the time lapse. Error bars represent SEMs from three independent experiments. ***, p < 0.001 by one-way ANOVA with correction for multiple comparisons. (G) Histogram of time to first appearance for each droplet. (H) Average intensity curves for FBs during the time lapse. Time 0 defined as the frame before first appearance of the droplet. Error bars represent SEMs from average droplet intensity values per time point over three independent experiments. Absolute intensity values are likely underestimated due to bleaching. Scale bars: 5 µmReferences
-
- Agarwal AK, Garg A. Genetic basis of lipodystrophies and management of metabolic complications. Annu Rev Med. 2006;57:297–311. - PubMed
-
- Bi J, Wang W, Liu Z, Huang X, Jiang Q, Liu G, Wang Y, Huang X. Seipin Promotes adipose tissue fat storage through the ER Ca2+-ATPase SERCA. Cell Metab. 2014;19:861–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

