A gold cyano complex in nitromethane: MD simulation and X-ray diffraction
- PMID: 25540462
- PMCID: PMC4267153
- DOI: 10.1016/j.cplett.2012.04.044
A gold cyano complex in nitromethane: MD simulation and X-ray diffraction
Abstract
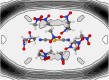
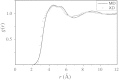
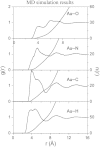
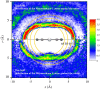
The solvation structure around the dicyanoaurate(I) anion (Au(CN)2-) in a dilute nitromethane (CH3NO2) solution is presented from X-ray diffraction measurements and molecular dynamics simulation (NVT ensemble, 460 nitromethane molecules at room temperature). The simulations are based on a new solute-solvent force-field fitted to a training set of quantum-chemically derived interaction energies. Radial distribution functions from experiment and simulation are in good agreement. The solvation structure has been further elucidated from MD data. Several shells can be identified. We obtain a solvation number of 13-17 nitromethane molecules with a strong preference to be oriented with their methyl groups towards the solute.
Figures



References
-
- Pyykko P. Gold Bull. 2004;37:136.
-
- Cui J.R., Zhang L.F. J. Hazard. Mater. 2008;158:228. - PubMed
-
- Wannakao S., Khongpracha P., Limtrakul J. J. Phys. Chem. A. 2011;115:12486. - PubMed
-
- Khongpracha P., Probst M., Limtrakul J. Eur. Phys. J. D. 2008;48:211.
-
- Huber S.E., Warakulwit C., Limtrakul J., Tsukuda T., Probst M. Nanoscale. 2012;4:585. - PubMed
LinkOut - more resources
Full Text Sources
