Use of systems pharmacology modeling to elucidate the operating characteristics of SGLT1 and SGLT2 in renal glucose reabsorption in humans
- PMID: 25540623
- PMCID: PMC4261707
- DOI: 10.3389/fphar.2014.00274
Use of systems pharmacology modeling to elucidate the operating characteristics of SGLT1 and SGLT2 in renal glucose reabsorption in humans
Abstract
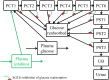
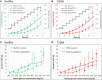
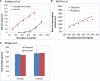
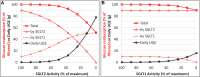
In the kidney, glucose in glomerular filtrate is reabsorbed primarily by sodium-glucose cotransporters 1 (SGLT1) and 2 (SGLT2) along the proximal tubules. SGLT2 has been characterized as a high capacity, low affinity pathway responsible for reabsorption of the majority of filtered glucose in the early part of proximal tubules, and SGLT1 reabsorbs the residual glucose in the distal part. Inhibition of SGLT2 is a viable mechanism for removing glucose from the body and improving glycemic control in patients with diabetes. Despite demonstrating high levels (in excess of 80%) of inhibition of glucose transport by SGLT2 in vitro, potent SGLT2 inhibitors, e.g., dapagliflozin and canagliflozin, inhibit renal glucose reabsorption by only 30-50% in clinical studies. Hypotheses for this apparent paradox are mostly focused on the compensatory effect of SGLT1. The paradox has been explained and the role of SGLT1 demonstrated in the mouse, but direct data in humans are lacking. To further explore the roles of SGLT1/2 in renal glucose reabsorption in humans, we developed a systems pharmacology model with emphasis on SGLT1/2 mediated glucose reabsorption and the effects of SGLT2 inhibition. The model was calibrated using robust clinical data in the absence or presence of dapagliflozin (DeFronzo et al., 2013), and evaluated against clinical data from the literature (Mogensen, 1971; Wolf et al., 2009; Polidori et al., 2013). The model adequately described all four data sets. Simulations using the model clarified the operating characteristics of SGLT1/2 in humans in the healthy and diabetic state with or without SGLT2 inhibition. The modeling and simulations support our proposition that the apparent moderate, 30-50% inhibition of renal glucose reabsorption observed with potent SGLT2 inhibitors is a combined result of two physiological determinants: SGLT1 compensation and residual SGLT2 activity. This model will enable in silico inferences and predictions related to SGLT1/2 modulation.
Keywords: SGLT; dapagliflozin; diabetes mellitus; glucosuria; renal glucose reabsorption; systems pharmacology model.
Figures



References
-
- Brown B. P., Hawley H. B., Hessen M. T. (2011). Magill's Medical Guide, Vol. 3: Fluids and Electrolytes—Kidneys Pasadena, CA: Salem Press, Incorporated.
-
- Calado J., Sznajer Y., Metzger D., Rita A., Hogan M. C., Kattamis A., et al. . (2008). Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol. Dial. Transplant. 23, 3874–3879. 10.1093/ndt/gfn386 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

