Dynamics of nutrient uptake strategies: lessons from the tortoise and the hare
- PMID: 25540674
- PMCID: PMC4270431
- DOI: 10.1007/s12080-010-0110-0
Dynamics of nutrient uptake strategies: lessons from the tortoise and the hare
Abstract
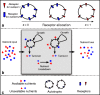
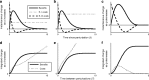
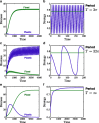
Many autotrophs vary their allocation to nutrient uptake in response to environmental cues, yet the dynamics of this plasticity are largely unknown. Plasticity dynamics affect the extent of single versus multiple nutrient limitation and thus have implications for plant ecology and biogeochemical cycling. Here we use a model of two essential nutrients cycling through autotrophs and the environment to determine conditions under which different plastic or fixed nutrient uptake strategies are adaptive. Our model includes environment-independent costs of being plastic, environment-dependent costs proportional to the rate of plastic change, and costs of being mismatched to the environment, the last of which is experienced by both fixed and plastic types. In equilibrium environments, environment-independent costs of being plastic select for tortoise strategies-fixed or less plastic types-provided that they are sufficiently close to co-limitation. At intermediate levels of environmental fluctuation forced by periodic nutrient inputs, more hare-like plastic strategies prevail because they remain near co-limitation. However, the fastest is not necessarily the best. The most adaptive strategy is an intermediate level of plasticity that keeps pace with environmental fluctuations, but is not faster. At high levels of environmental fluctuation, the environment-dependent cost of changing rapidly to keep pace with the environment becomes prohibitive and tortoise strategies again dominate. The existence and location of these thresholds depend on plasticity costs and rate, which are largely unknown empirically. These results suggest that the expectations for single nutrient limitation versus co-limitation and therefore biogeochemical cycling and autotroph community dynamics depend on environmental heterogeneity and plasticity costs.Electronic supplementary material The online version of this article (doi:10.1007/s12080-010-0110-0) contains supplementary material, which is available to authorized users.
Keywords: Biogeochemistry; Co-limitation; Dynamics; Ecosystem theory; Nutrient limitation; Plasticity; Strategies.
Figures



References
-
- Ahlgren G. Growth of Oscillatoria agarhii in chemostat culture 3. Simultaneous limitation by nitrogen and phosphorus. Eur J Phycol. 1985;20(3):249–261. doi: 10.1080/00071618500650261. - DOI
-
- Alon U. An introduction to systems biology: design principles of biological circuits. Boca Raton: Chapman and Hall; 2006.
-
- Ballantyne F, Menge DNL, Weitz JS. A discrepancy between predictions of saturating nutrient uptake models and nitrogen-to-phosphorus stoichiometry in the surface ocean. Limnol Oceanogr. 2010;55(3):997–1008. doi: 10.4319/lo.2010.55.3.0997. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
