Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression
- PMID: 25540771
- PMCID: PMC4261134
- DOI: 10.3389/fonc.2014.00359
Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression
Abstract
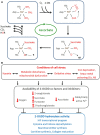
Ascorbate is a specific co-factor for a large family of enzymes known as the Fe- and 2-oxoglutarate-dependent dioxygenases. These enzymes are found throughout biology and catalyze the addition of a hydroxyl group to various substrates. The proline hydroxylase that is involved in collagen maturation is well known, but in recent times many new enzymes and functions have been uncovered, including those involved in epigenetic control and hypoxia-inducible factor (HIF) regulation. These discoveries have provided crucial mechanistic insights into how ascorbate may affect tumor biology. In particular, there is growing evidence that HIF-1-dependent tumor progression may be inhibited by increasing tumor ascorbate levels. However, rigorous clinical intervention studies are lacking. This review will explore the physiological role of ascorbate as an enzyme co-factor and how this mechanism relates to cancer biology and treatment. The use of ascorbate in cancer should be informed by clinical studies based on such mechanistic hypotheses.
Keywords: TET enzymes; ascorbate; cancer; hydroxylation; hypoxia-inducible factor-1; tumor microenvironment; vitamin C.
Figures
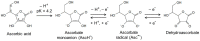

References
-
- Ball GFM. Ascorbic acid: physiology. In: Caballero B, editor. Encyclopedia of Food Sciences and Nutrition. Oxford: Academic Press; (2003). p. 324–32.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

